Abstract
Aims: Mechanical left ventricular (LV) unloading may reduce infarct size when combined with primary percutaneous coronary intervention (PCI) in patients with ST-elevation myocardial infarction (STEMI). The Impella LP2.5 is a novel percutaneous left ventricular assist device. Although the short-term safety and feasibility of this device have been demonstrated, the long-term effects are unknown. The purpose of the current study was to evaluate the long-term effects of the Impella LP2.5 support on the aortic valve and left ventricular ejection fraction (LVEF).
Methods and results: In 2006, 10 patients with anterior STEMI received 3-day support with the Impella LP2.5 after PCI. The control group consisted of 10 comparable patients, treated according to routine care. For the current study, echocardiography was performed and adverse events were recorded. Mean duration of follow-up was 2.9±0.6 years in the Impella group and 3.0±0.3 years in the control group. No differences in aortic valve abnormalities and LVEF were demonstrated between the groups; nevertheless, LVEF increase from baseline was significantly greater in Impella-treated patients (23.6±8.9% versus 6.7±7.0%, P=0.008).
Conclusions: Three-day support with the Impella LP2.5 is not associated with adverse effects on the aortic valve at long-term follow-up. LVEF was similar in both groups; however, recovery was significantly greater in the Impella group.
Introduction
Primary percutaneous coronary intervention (PCI) has been demonstrated to reduce infarct size1 and improve prognosis in patients with ST-elevation myocardial infarction (STEMI), when compared with thrombolytic therapy2. When STEMI is complicated by haemodynamic instability or cardiogenic shock (CS), in-hospital mortality rates remain high3,4 despite early revascularisation5,6. Additionally, profound left ventricular dysfunction and subsequent disability remain an important problem in survivors of STEMI complicated by CS. Mechanical left ventricular (LV) support may further reduce infarct size by means of LV unloading, in addition to reperfusion by primary PCI. The intra-aortic balloon pump (IABP) was developed in the 1960s as the first percutaneous left ventricular support device7. However, as we recently demonstrated in a meta-analysis of available evidence for IABP therapy, its use is not clearly associated with improved survival or residual left ventricular function8. In the experimental setting, LV unloading by a percutaneous left ventricular assist device (LVAD) has been demonstrated to further reduce infarct size when combined with reperfusion9,10. The Impella LP2.5 (Abiomed Europe, Aachen, Germany) is a novel catheter-mounted micro-axial intracardiac left ventricular assist device. We demonstrated safety and feasibility of 3-day Impella support in haemodynamically stable STEMI patients with large anterior myocardial infarction in a small non-randomised pilot study (‘MACH 2’). In this study, we also observed a beneficial effect on LV function11. However, in the setting of prolonged support with the Impella LP2.5 device, long-term safety with regard to the occurrence of aortic valve damage and efficacy with regard to the functional status and the left ventricular function are currently unknown.
Methods
Patient population and pilot study design
The original study was a single-centre, prospective cohort pilot study, designed to evaluate the safety and feasibility of left ventricular unloading with the Impella LP2.5 device in anterior STEMI patients treated with primary PCI with a 4-month follow-up after therapy. The primary endpoint was to demonstrate safety and feasibility. The secondary endpoint was to explore LV recovery. Patients that were included in our pilot study were between 30 and 80 years of age, had had a first anterior STEMI and presented within six hours of symptom onset. Patients who were in cardiogenic shock, had a blood transfusion in the 24 hours before presentation, had known haemoglobin diseases such as sickle cell or thalassaemia, stroke or transient ischaemic attack within four weeks before presentation or had serious known concomitant disease with a life expectancy of less than one year were excluded from the study. Other exclusion criteria were related to the ability to insert the Impella device and included the presence of a mural thrombus in the left ventricle, the presence of severe peripheral arterial disease, the presence of aortic stenosis with an orifice area of 1.5 cm2 or less and the presence of a mechanical aortic valve. Ten patients were treated with the Impella LP2.5 device, whereas a concurrent group of 10 consecutive patients meeting all the eligibility criteria, but who did not receive Impella support, served as a control group. Baseline characteristics from both the Impella group and the control group have been described previously11 and are displayed in Table 1.
Impella LP2.5
The Impella LP2.5 device (Abiomed Europe GmbH, Aachen, Germany) has been described previously11,12. It is a catheter-mounted (9 Fr), micro-axial rotary blood pump (12 Fr), designed for short-term mechanical circulatory support, which is inserted through the femoral artery and positioned across the aortic valve into the left ventricle using fluoroscopy. The driving console of the pump allows management of pump speed (by 9 gradations) and displays the pressure difference between inflow and outflow, which gives an indication for pump position. The device provides a flow of up to 2.5 L/min at its maximal rotation speed of 50.000 rpm, through expelling blood from the left ventricle into the ascending aorta. The Impella LP2.5 device was inserted immediately after PCI. Patients were assigned to receive 72 hours of Impella treatment, including at least 48 hours of active support. Active support is defined as Impella support level P>5, preferably P7 (higher support levels are usually not achieved for a long period). Between 48 and 72 hours after PCI, Impella assistance was lowered until P2 (or P3 if diastolic pressure >80 mmHg) for the last 8-10 hours before removal. After 72 hours, the Impella device was removed. Haemostasis after pump removal was achieved by manual compression.
Data collection
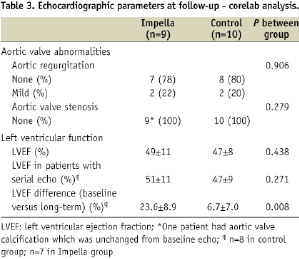
For the current study, all pilot study participants were contacted through their general practitioners. One patient from the Impella group required HeartMate II implantation within four months after enrolment. This patient had been excluded from the initial pilot study analyses and was excluded from the present analysis as well. Therefore, 19 patients participated in the follow-up study protocol. Adverse events, current medication use and the presence of cardiac risk factors were recorded. Laboratory measurements were performed, including haemoglobin, glucose, creatinine and N-terminal-pro brain natriuretic peptide (NT-pro-BNP). Two-dimensional echocardiography was performed in standard parasternal long and short axis views and apical five-, four- and two-chamber views, including colour Doppler and tissue Doppler imaging. All echocardiograms were subsequently analysed by an independent core-lab (Cardialysis BV, Rotterdam, The Netherlands). Valve abnormalities were assessed according to the ACC/AHA guidelines13 and defined according to the following criteria: aortic regurgitation (AR) was considered to be mild when any central jet was present with a width <25% of the left ventricular outflow tract (LVOT). Moderate AR was present when signs of AR were greater than mild, but did not meet the criteria for severe AR. Severe AR was defined as a jet width of >65% of LVOT diameter.
Aortic valve stenosis (AS) was considered to be mild when the aortic valve area was 1.5 cm2, the mean gradient was <25 mm Hg, or jet velocity was <3.0 m/s. Moderate AS was defined as a valve area from 1.0 to 1.5 cm2, a mean gradient 25 to 40 mm Hg, or a jet velocity of 3.0 to 4.0 m/s and severe AS was present when the aortic valve area was <1.0 cm2, the mean gradient >40 mm Hg, or jet velocity was >4.0 m/s. Left ventricular ejection fraction (LVEF) was assessed quantitatively according to the biplane Simpson’s method.
Statistical analysis
Data were analysed using the statistical “Package for the Social Sciences” (SPSS Inc., Chicago, IL, USA; version 16.0.2). Continuous data are presented as mean ±standard deviation (SD) (median and quartiles for skewed variables). Categorical data are presented as percentages. All p values <0.05 were considered statistically significant. Differences between groups were tested using the χ2 test for categorical variables (Fisher’s exact test as appropriate) and the unpaired Student’s t-test for normally distributed continuous variables (Mann-Whitney U test as appropriate). Furthermore, the difference in LVEF percentage points from baseline to long-term follow-up was tested between groups using the Mann-Whitney U test. In addition, ANCOVA analysis was performed to evaluate the predictive value of baseline LVEF and Impella treatment with regard to long-term LVEF.
Results
Baseline characteristics
Baseline characteristics of both groups are displayed in Table 1. Although differences between groups are not statistically significant, patients in the Impella group tended to have higher NT-pro BNP values, higher baseline and peak creatine kinase –MB (CK-MB) levels and higher high-sensitivity C-reactive protein (hs-CRP) levels. Additionally, patients in the Impella group had a significantly lower baseline LVEF when compared with the control group (28%±3 versus 40%±7, P<0.05).
Safety
No signs of adverse effects on the aortic valve had been observed during Impella support or after four months of follow-up. Four Impella-treated patients experienced groin bleeding requiring transfusion, compared with two in the control group. However, after implementing a more stringent institutional heparin protocol, oozing was no longer an important issue. Haemolysis (free-haemoglobin levels ≥10 mg/dl) occurred only within the first 24 hours of support. After 24 hours, free-haemoglobin levels normalised quickly, as depicted in Figure 1.
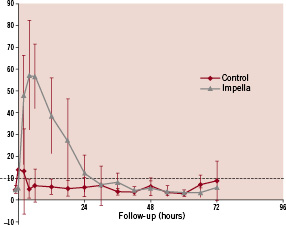
Figure 1. Serial free haemoglobin levels in the Impella and control groups. 10 mg/dL denoting the upper limit of normal and 50 mg/dL denoting severe haemolysis.
There were no other device-related adverse events. None of the patients in either group experienced any major adverse cardiac or cerebral events during hospital admission and four month follow-up.
Haemodynamic effects during Impella support
Impella insertion was successful in all cases. Median time for placement was 11 minutes. After insertion and subsequent maximisation of pump performance (mean maximum flow: 2.2±0.1 L/min) an immediate increase in cardiac output (4.4±0.3 l/min to 4.9±0.5 l/min, n=5, p=NS) and decrease in pulmonary capillary wedge pressure (24.3±2.4 mmHg to 17.3±0.4 mm Hg, n=5, p<0.05) was observed.
Patient characteristics at follow-up
Mean duration of follow-up was 2.9±0.6 years in the Impella group and 3.0±0.3 years in the control group. In the Impella group, one patient was lost to follow-up as described in the methods section. Patients in the Impella group were older (63±9 years versus 61±10 years in the control group) and more often male (89% versus 60%), although differences were not statistically significant. Groups did not differ with regard to current risk profile, except for smoking and hypertension, which were more prevalent in the Impella group. Current medication use did not differ between groups. No significant differences were observed between groups with regard to NT-pro BNP, glucose, creatinine and haemoglobin values. With the exception of target vessel revascularisation for in-stent restenosis in one patient in the Impella group, no adverse events occurred. Patient characteristics, laboratory measurements and adverse events are displayed in Table 2.
Echocardiography at follow-up
Mild aortic regurgitation was present in two patients in both groups (P=ns). One patient in the Impella group had mild aortic cusp calcification, which was present from the index event before Impella insertion. LVEF was not significantly different between groups. In patients who had echocardiograms available on all time points from baseline to long-term follow-up, the absolute increase in LVEF was 6.7±7.0% in the control group and 23.6±8.9% in the Impella group (P=0.008) when compared to baseline. ANCOVA analysis was used to adjust for baseline LVEF; the results of ANCOVA analysis were in accordance with the simple comparison of absolute percentage differences. Echocardiographic parameters are displayed in Table 3.
Serial LVEF measurements are displayed in Figure 2.
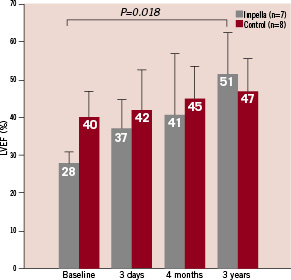
Figure 2. Serial evaluation of left ventricular ejection fraction (LVEF). Vertical bars show mean values±SD for paired LVEF data.
Discussion
Aortic valve
The current data from our pilot study follow-up show that 3-day support with the Impella LP2.5 does not have any late adverse effects on the aortic valve, as no differences were observed between groups with regard to aortic valve abnormalities11. Short-term effects of Impella support on the aortic valve have been investigated in elective PCI12,14,15. Moreover, in our pilot study, effects of 3-day support with the device were investigated in the setting of STEMI. Echocardiographic core lab adjudicated follow-up was performed up to four months11. Adverse effects on the aortic valve have not been demonstrated in any of these studies. Limited data are available with regard to long-term follow-up. Burzotta et al have reported on follow-up in elective high-risk PCI up to one year, however, echocardiographic data were only collected for up to six months and data on aortic valve abnormalities are lacking16. Data from our current study support the short- and long-term safety of Impella treatment with regard to effects on the aortic valve.
Left ventricular function
Left ventricular function was not significantly different between the Impella group and control group patients after three years of follow-up. However, when evaluating the recovery of left ventricular function compared to baseline, patients in the Impella group had a significantly greater LVEF recovery than control group patients. LVEF at baseline was significantly lower in the Impella group, whereas no significant difference remained at four months and long-term follow-up. Although LV recovery has been investigated at several time points, data on long-term LVEF (>6 months of follow-up) after myocardial infarction are sparse. Halvorsen et al have investigated LVEF and infarct size after a mean follow-up duration of 20 months. They observed a well preserved LVEF, which was not significantly different from mean LVEF at discharge and at six weeks follow-up17. However, discharge LVEF in this study was 56%, which may have precluded additional recovery. Besides primary PCI, which effectively reduces infarct size and mortality when compared to thrombolysis, left ventricular unloading has been hypothesised to have an additional effect on infarct size1,2. This has been demonstrated in the experimental setting9,10. The acute haemodynamic effects of Impella support, including an increase in cardiac output and a decrease in PCWP, suggest an effect on LV loading conditions. Moreover, we recently described the unloading effect of the Impella LP2.5, which was demonstrated by direct PV-loop measurements within this pilot study18. With regard to its use in acute myocardial infarction patients and longer durations of support, the Impella LP2.5 has been investigated in the ISAR-SHOCK trial by Seyfarth et al19. In this study, patients with an acute myocardial infarction, complicated by cardiogenic shock, were randomised to Impella LP2.5 versus IABP. The primary endpoint was cardiac index after 30 minutes of support, which was higher in Impella-supported patients. This study was included in a recent meta-analysis as well20, evaluating the effect of percutaneous left ventricular assist devices. However, as the other two studies in this meta-analysis concern the TandemHeart percutaneous left ventricular assist device, its value with regard to discussing the current results may be limited. Both short- and long-term results from our pilot study suggest a possible benefit of Impella support, although these results should be considered hypothesis-generating. Nevertheless, encouraged by our pilot study results, we are currently conducting a trial of IABP versus Impella LP2.5 in cardiogenic pre-shock patients (IMPRESS in STEMI, NTR1079, www.trialregister.nl), evaluating LVEF as a primary endpoint.
Study limitations
Our current long-term results are derived from follow-up to a non-randomised pilot study, which may have induced some selection bias. Although differences were not statistically significant at baseline, a trend towards worse baseline clinical condition was observed in Impella-treated patients, in accordance with daily clinical practice for percutaneous mechanical circulatory support. However, current results are similar between groups, suggesting more profound recovery in Impella-treated patients. Furthermore, sample size was small, with 10 patients included in each group at baseline, 10 patients left in the control group and nine patients left in the Impella-group at follow-up. Moreover, some additional data are missing in both groups, rendering the sample size even smaller with regard to some parameters. Paired analysis of LVEF, for example, was possible in eight patients in the control group and seven patients in the Impella-treated group. Furthermore, haemodynamic data were only available in five of the Impella-treated patients. Notwithstanding the small sample size however, we still consider our results to be encouraging, although larger-scale randomised studies are needed to investigate the effect of Impella support on left ventricular function.
Conclusion
Three-day support with the Impella LP2.5 after STEMI is safe with regard to long-term effects on the aortic valve. Left ventricular function is similar in Impella-treated patients and control group patients at long-term follow-up. Nevertheless, recovery of LVEF when compared to baseline is significantly greater in patients who had received 3-day Impella support after STEMI.
Acknowledgements
We would like to thank Mrs. Denise Samson, senior sonographer, Academic Medical Centre, Amsterdam, The Netherlands, for acquiring high-quality echocardiographic images in all patients.