Abstract
The contribution of carotid baroreceptor feedback in preventing or potentially contributing to the essential hypertensive cascade is poorly understood. It is clear the carotid sinus nerve action potentials are triggered by carotid bulb stretch rather than pressure and are only sustained during pulsatile increases in pressure. In addition, the carotid baroreceptor negative feedback is gradually extinguished in hypertension patients (a phenomenon known as “resetting”). We report a case of significant reduction in blood pressure in a patient with true resistant hypertension after change in the carotid bulb pulsatile strain patterns following the implant of an intravascular prosthesis.
Introduction
Hypertension (HTN) impacts on nearly one third of the general population. Poor HTN control can be driven by patients’ non-compliance, untreated secondary causes, or inadequate medical treatment. However, as many as 30% of hypertensive patients who are on three medications or more fail to reach goal blood pressures and fall into the category of “resistant hypertension”1. Patients with resistant HTN are at higher risk of cardiovascular events as compared to patients with controlled hypertension1. Guidelines emphasise the importance of maximal medical therapy, adequate work-up for reversible causes, and availability of counselling on the importance of medication compliance1,2. However, it is anticipated that millions of patients could still benefit from an alternative treatment2. A definitive pharmaceutical solution has remained elusive, leading to exploration of invasive alternatives designed to sever autonomic excitatory pathways (i.e., renal denervation) or stimulate inhibitory reflexes (i.e., electrical stimulation of the carotid sinuses)1,3. Focus on the autonomic nervous system seems justified as excitatory sympathetic imbalance worsens with HTN severity.
We present a case of a novel passive carotid baroreceptor-stimulating device implantation in a patient with true drug-resistant hypertension.
Device background
The design of the implant (i.e. the MobiusHD™ device, Vascular Dynamics, Inc., Mountain View, CA, USA) was based on several key known principles of baroreceptor physiology, anatomy and function. First, it is known that the carotid sinus nerve action potential is triggered by carotid body stretch rather than pressure sensing. In addition, sustained activation of the carotid baroreceptor reflex requires pulsatile stretch, which explains why carotid stents do not have a long-term impact on blood pressure4. Also, baroreceptor “resetting” takes place in hypertensive patients reducing or eliminating an important negative sympathetic feedback loop, potentially adding to the hypertensive cascade. Finally, sustained blood pressure reduction can be seen with carotid sinus electrical stimulation, suggesting that successful amplification of the carotid baroreceptor signals should yield a sustainable haemodynamic change4.
Working in the framework of the need to increase carotid sinus stretch while maintaining pulsatility, a simple strain equation was used to define the ideal implant architecture. It was found that increased vessel wall strain could be achieved when the axial geometry of an elastic tubular structure is changed to a non-circular cross-section with an intravascular or extravascular scaffold (Figure 1A)4. This led to the development of a self-expanding nitinol prosthesis as shown in Figure 1B. The device concept was then vetted with finite element and laminar flow analysis, extensive benchtop testing, cadaver studies and animal studies prior to embarking on this first-in-man study5.
The case presented is the first case of the CALM-FIM_EUR trial (ClinicalTrials.gov, NCT01911897). The trial is a 20-patient non-randomised multicentre safety study sponsored by Vascular Dynamics, Mountain View, CA, USA. The study involves two mirror protocols with 10 patients at six US sites and 20 patients at five European sites. The device delivery catheter is a 5.8 Fr, monorail system that allows for recapture and repositioning.
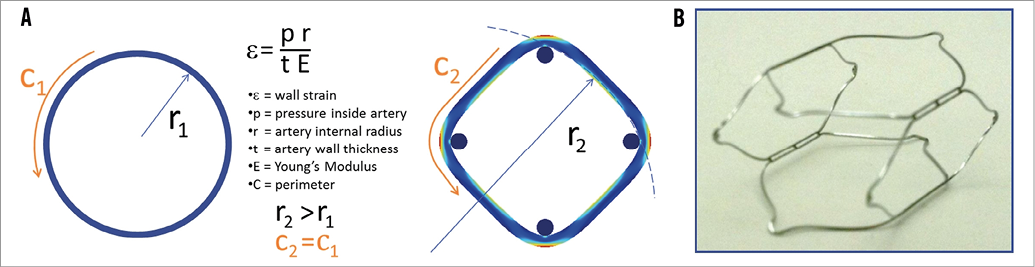
Figure 1. Reshaping the artery to a non-circular cross-section results in a dramatic increase in radius. Note the circumference of the circle C1 remains the same as the perimeter of the square C2. The other variables are not changed with the implant, so significant increase in wall strain should be expected (A). B) Non-constrained self-expanding nitinol prosthesis.
Case report
A 53-year-old woman with a history of hyperhomocysteinaemia and hypothyroidism presented with stage 3 essential HTN and associated secondary organ damage (grade 2 hypertensive retinopathy, mild renal insufficiency [glomerular filtration rate 54 ml/min/1.73 m2] and mild proteinuria 0.30 g/l). The patient was diagnosed with hypertension in 2008 and was subsequently treated with various antihypertensive drug regimens. At that time work-up for secondary causes was negative. Magnetic resonance angiography (MRA) in 2009 revealed no significant stenosis in the renal arteries, and in 2010 the patient underwent bilateral renal denervation with the indication being drug-resistant HTN. However, the office and home blood pressures remained high, as did ambulatory 24-hour blood pressure monitoring. Despite six different antihypertensive drugs (labetolol 400 mg four times daily; furosemide 80 mg twice daily; spironolactone 50 mg three times daily; and valsartan 160 mg, hydrochlorothiazide 25 mg and amlodipine 10 mg in a combination tablet once daily), the blood pressure remained out of control (Figure 2). In December 2013, the patient was enrolled in the CALM-FIM_EUR study for implantation of the MobiusHD™ device. True resistant HTN was documented by 24-hour blood pressure monitor (90217 Ultralite Ambulatory Blood Pressure Monitor; Spacelabs Healthcare, Snoqualmie, WA, USA), office blood pressure evaluation, medication compliance log and twice daily home blood pressure with digital acquisition (BP791IT Blood Pressure Monitor; OMRON Corporation, Kyoto, Japan). Carotid duplex prior to enrolment showed no stenosis, and the MRA suggested the aortic arch and great vessels were free of significant disease. Through a right transfemoral access, with the support of an 8 Fr multipurpose guiding catheter and 0.014” wire, the MobiusHD device size B (i.e., size 6.25-9.00 mm) was deployed into the right carotid sinus (Figure 3, Moving image 1, Moving image 2). On the day of the procedure, blood pressure response was mild (average 150 over 90 mmHg); however, the next day and during follow-up blood pressure dropped to normal values.
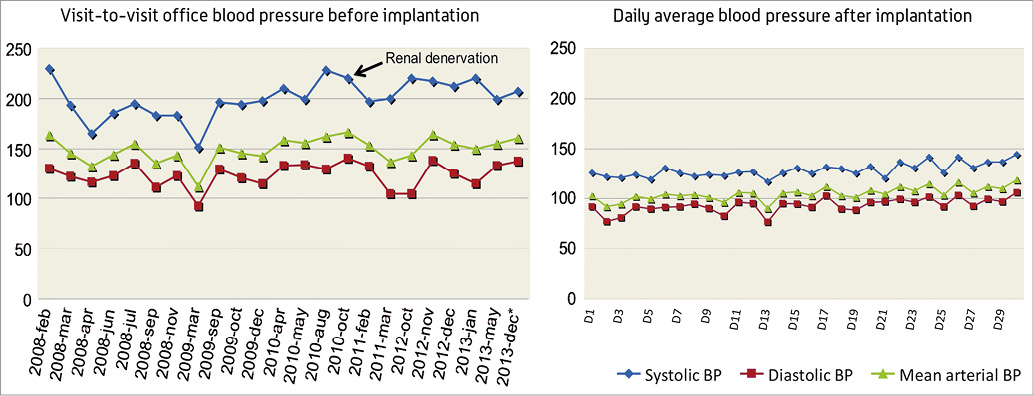
Figure 2. Follow-up of the blood pressure prior to and after device implantation. * Denotes the average of the blood pressure measures the day before the MobiusHD device implantation.
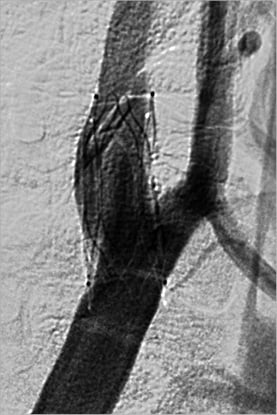
Figure 3. Angiography of the right carotid artery after implantation of the device.
A 30-day follow-up with twice daily ambulatory blood pressure monitoring showed an average blood pressure of 128 over 93 mmHg (Figure 2). Ten weeks after implantation, an ambulatory 24-hour blood pressure monitoring revealed normal blood pressure with a mean of 113 over 70 mmHg during the day and a mean of 107 over 60 mmHg during the night. This compared favourably to the pre-procedure 24-hour readings of 183 over 114 mmHg during the day and a mean of 181 over 109 mmHg during the night. After the device implantation no significant changes in heart rate were observed (average heart rate of 77 bpm and 68 bpm before vs. after the procedure, respectively). During follow-up after the device implantation, labetalol was stopped, and furosemide and spironolactone doses were reduced. Her present medications are furosemide 40 mg twice daily; spironolactone 50 mg twice daily, and valsartan 160 mg, hydrochlorothiazide 25 mg and amlodipine 10 mg in a combination tablet once daily.
Discussion
Carotid bulb modification with the MobiusHD device resulted in impressive hypertension control in this case report. The findings in this case are noteworthy since this is the first European experience with a carotid baroreceptor modulation device and blood pressure response was so dramatic. The clinical findings are compelling since the patient had undergone renal denervation in the past without blood pressure improvement.
Generally, little can be concluded from a single case. However, the initial dramatic clinical results in our case were very gratifying, but additional follow-up will be needed to ensure durability of the blood pressure lowering effect. In theory, there could be decay of therapeutic effect as the carotid bulb remodels or if secondary resetting occurs. In addition, it will be important to confirm these results in the CALM-FIM trial before embarking on large randomised studies. The lessons learnt from recent renal denervation and renal stent trials have helped us understand better the importance of randomised trials, and of sham treatment in the control group in the non-pharmacologic treatment of resistant hypertension3. Finally, the risks of carotid transcatheter instrumentation will need to be counterbalanced by documented reproducible results in true resistant HTN patients.
Irrespective of the trial outcome, the dramatic blood pressure changes in this case may stimulate interest in understanding the role of carotid sinus baroreceptor resetting better in the contribution to resistant hypertension and lead to exploration of other therapeutic alternatives.
Conflict of interest statement
M. Bates is a consultant for Vascular Dynamics. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Pre-implantation angiogram of the right carotid artery.
Moving image 2. Post-implantation angiogram of the right carotid artery.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Pre-implantation angiogram of the right carotid artery.
Moving image 2. Post-implantation angiogram of the right carotid artery.

