Abstract
The contribution of sympathetic activation in the development of hypertension is supported by early experimental evidence based on surgical denervation of sino-aortic baroreceptors or lesions of the central relay station of the baroreflex, the nucleus tractus solitarii1. Disruption of this area of the brain was associated with an immediate increase in blood pressure1. Sympathetic overactivity can also be triggered by impairment of the inhibitory function physiologically exerted by reflexogenic areas (arterial baroreceptors, cardiopulmonary receptors, and chemoreceptors) on adrenergic drive2. Metabolic and humoral mechanisms are also thought to be involved in the development and progression of hypertension-related sympathetic overdrive3.
The role of baroreceptors in the development of hypertension
The baroreceptors of the carotid sinus sit at the bifurcation of the common carotid artery and are mechanoreceptors that respond to vascular distention. The carotid baroreflex is an important component in the control of both short-term and long-term fluctuations in blood pressure. The reflex is initiated by stimulation of stretch-sensitive afferent nerve endings found within the common carotid artery bifurcation3. The stimulation of these receptors in response to vascular distention from either elevated BP or increased intravascular volume acts to dampen increases in blood pressure. In response to a sensed “stretch,” the baroreceptor sends a signal that travels from the carotid sinus nerve to join cranial nerve IX (CN IX), eventually signalling the nucleus tractus solitarius in the medulla4. Ultimately, this leads to an inhibition of sympathetic output, along with a decrease in the heart rate, cardiac contractility as well as the release of renin-angiotensin-aldosterone system (RAAS) and ADH, which serves to reduce intravascular volume and tone4, Figure 1.
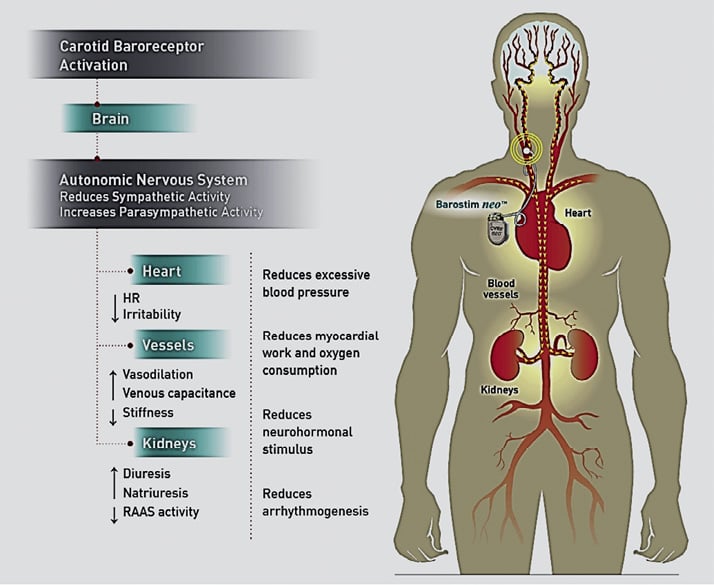
Figure 1. The effect of baroreceptors on the maintenance of blood pressure regulation.
Baroreceptor activation therapy (BAT)
Direct stimulation of the baroreceptor should lower blood pressure. Early carotid sinus stimulating devices were bulkier and more frequently a cause of postural hypotension, localised nerve and soft tissue irritation. They also commonly required adjustment of electrical stimulation5.
The original Rheos Hypertension System is (Rheos System; CVRx Inc., Minneapolis, MN, USA) an implantable carotid sinus baroreceptor stimulator targeted to treat patients with resistant hypertension. It is comprised of three components: 1) Rheos Implantable Pulse Generator; 2) bilateral Rheos Carotid Sinus Leads (CSL); and 3) Rheos Programmer System6, Figure 2. The generator itself is comprised of a battery and circuit system that activates the receptors with variable amounts of energy in a temporally varying pattern via the carotid leads. The Rheos Programmer System is a computer-based programming system that allows noninvasive communication with and control of the generator via radiofrequency coupling. The generator is directly connected to the leads, which measure 50 cm in length.
The Rheos Feasibility Trial was a United States-based study looking at a series of 10 patients with resistant hypertension after implantation of the Rheos Baroreflex Hypertension Therapy (BHT) System7. Eligibility was limited to patients with resistant hypertension, defined as SBP greater than 160 mmHg despite receiving maximal appropriate doses of three or more antihypertensive medications, including a diuretic. Inclusion criteria included maximisation of antihypertensive treatment, elimination of secondary causes of hypertension, exclusion of baroreflex dysfunction, and absence of carotid disease. Three months of device therapy resulted in clinically and statistically significant reductions in office cuff SBP (–22 mmHg) and DBP (–18 mmHg). These findings were confirmed by concurrent reductions of mean 24-hour ambulatory pressure: SBP (–14 mmHg) and DBP (–9 mmHg). Similar results were noted in the European trials7. No episodes of symptomatic hypotension, bradycardia, heart block or adverse renal effects were reported.
The Device-Based Therapy of Hypertension (DEBuT-HT) trial was a European-based multicentre study looking at the safety and efficacy of BHT in severely hypertensive patients, conducted by a consortium of nine European institutions8. The inclusion criteria were the same with the Rheos Feasibility trial. Study patients continued to use a mean of 4.6 antihypertensive medications; this was not changed from their mean use of 4.8 medications at baseline. From the 45 patients enrolled in the study, 18 patients successfully completed a mean duration of 58±6 months of chronic therapy with the Rheos device. BP and HR were compared at baseline, three months, one year, two years, and three years. In total, 72% of patients were able to achieve at least a 30 mmHg sustained drop in SBP at three years, with 67% of patients measuring SBP <140 mmHg at that time. The mean reduction in DBP was –30 mmHg and the reduction in heart rate was –5 beats per minute by three years. In addition, chronic baroreceptor stimulation caused sustained changes in HR variability and heart turbulence, consistent with inhibition of sympathetic activity and an increase in parasympathetic activity9.
The DEBuT-HT study demonstrated that the Rheos system resulted in sustained BP reduction in patients with resistant hypertension. Moreover, the device and the technique were safe, seven out of 42 subjects (17%) experienced a procedure-related adverse event and one a device-related event. Prior to activation three subjects had the device explanted due to infection.
Finally, The Rheos Pivotal Trial10 evaluated BAT for resistant hypertension in a double-blind, randomised, prospective, multicentre, placebo-controlled Phase III clinical trial. This trial of 265 subjects with resistant hypertension were implanted and subsequently randomised (2:1) one month after implantation. Subjects received either BAT (Group A) for the first six months or delayed BAT initiation following the six-month visit (Group B). The main enrolment criterion was resistant HTN defined as two office cuff readings of SBP ≥160 mmHg within three months of enrolment. Confirmation of the resistant hypertension diagnosis required 24-hour average ambulatory SBP ≥135 mmHg and ≥1 month of ≥3 concomitant antihypertensive medications.
Outpatient office BP was assessed per protocol utilising a standardised, automated device that was programmed to take six measurements at 1-min intervals and report the average of the last five of these measurements, which were taken with the investigator not in the room. The five co-primary endpoints were: 1) acute SBP responder rate at six months; 2) sustained responder rate at 12 months; 3) procedure safety; 4) BAT safety; and 5) device safety.
From the 265 patients enrolled in the study, 264 patients (99%) successfully completed a mean duration of 21±8 months of chronic therapy with the Rheos device. The trial did not meet two of the five pre-specified co-primary endpoints, i.e., short-term safety and short-term efficacy. BAT yielded a decrease in SBP of 26 mmHg for Group A and 17 for Group B at six months and a decrease of 35 for Group A and 33 for Group B at 12 months. At 12 months, the SBP of 81% of subjects had dropped at least 10 mmHg from pre-implant. This responder group experienced an average SBP drop of 44 mmHg, and 63% of these subjects reached SBP of <140 mmHg. Heart rate was significantly reduced among responders in group A but not in group B or among non-responders in either group.
There were seven deaths (four occurring during the initial 12 months of follow-up and an additional three during long-term follow-up). None of these deaths were related to either the procedure or the device. The main procedure related side effects were associated with carotid sinus lead placement. Thus, this study provides additional evidence for safety of BAT. It also demonstrates clinically significant and sustained SBP reductions by the use of the Rheos device.
Following completion of the randomised Rheos Pivotal Trial, patients participated in open-label, non-randomised follow-up to assess safety and efficacy of BAT11. A significant percentage of patients who underwent device implantation (76%) qualified as clinically significant responders. Among long-term responders receiving BAT, the mean blood pressure drop was 35/16 mmHg. Medication use was reduced at the end of the randomised phase and remained lower through the follow-up period. Among responders, 55% achieved goal blood pressures (<140 mmHg or <130 mmHg in diabetes or kidney disease). This blood pressure reduction or goal achievement was maintained over long-term follow-up of 22 to 53 months11.
A recent trial of 322 patients started on baroreflex activation therapy early (one month, n=236) or late (six months, n=86) evaluated kidney function after device implantation12. The study showed a modest, insignificant decrease in estimated glomerular filtration rate (eGFR). This eGFR decrease correlated to a drop in blood pressure. There was no further or long-term reduction in eGFR. A non-significant reduction of urine albumin/creatinine ratio was also observed only in the late device activation group12.
Major limitations of the previous Rheos trials include the need for bilateral surgical placement of the electrodes around the carotid bifurcation and battery replacement after a few years. The new NEOS device requires much less surgical time with unilateral placement of a single electrode and a battery with a four to five year life span, Figure 2. A randomised blood pressure efficacy trial has just started in the United States with the new device for Food and Drug Administration labelling.
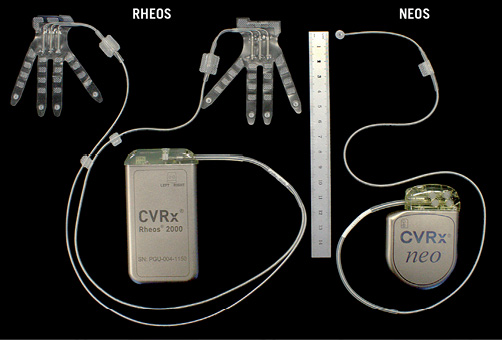
Figure 2. The original (RHEOS) and the most recent (NEOS) baroreceptor activating devices.
Taken together these data indicate that BAT offers important advantages over conventional pharmacologically based strategies for treatment resistant hypertension. The effectiveness of this device may be assessed immediately after implantation unlike that of renal denervation. Moreover, the device at this stage is implantable in people with kidney disease and apparently does not have adverse renal effects. It clearly serves as a possible intervention for people with resistant hypertension who have failed pharmacological treatment. It also serves as an option for those who may not qualify for renal denervation.
Conflict of interest statement
G. Bakris is the co-principal investigator of the Symplicity HTN-3 trial (Medtronic) and a consultant to CVRx, Takeda, AbbVie, Daiichi-Sankyo, Janssen and Relapsya. A. Briasoulis has no conflicts of interest to declare.

