Abstract
Our aim was to highlight the very recent and rapid expansion of a number of contemporary, pioneering and projected interventional approaches for resistant hypertension. Our discussions of each approach centre on a new model of the cardiovascular system that emphasises the importance of the sympathetic nervous system, its differential control and reflex activation. Four interventional therapeutic strategies are discussed including targeted sympathectomies and carotid body ablation. Harnessing endogenous homeostatic control systems looks promising as a means to correct autonomic balance. The challenge will be in deciding which interventional approach should be selected.
Introduction
Hypertension has traditionally been viewed as the downstream consequence of primary renal dysfunction that results in sodium and water retention1. Within this framework, hypertension associated with chronic activation of the sympathetic nervous system would be caused by increased activity of the renal sympathetic nerves and sodium retention specifically, leading to blood volume overload and consequential cardiac output elevation. However, a new conceptual paradigm suggests that sympathetic nervous system hyperactivity may cause hypertension independent of primary renal pathology2. In contrast to the classical Guyton-Coleman model1 which dictates primary impairment of the “pressure-natriuresis mechanism” as the single cause of all forms of hypertension, this alternative model assumes that natriuresis is regulated independently of arterial pressure and that blood volume distribution is determined by sympathetic regulation of arterial resistances of different vascular beds (which include the kidney) in the body2. This we refer to as the “Sympathetic Hypothesis of Hypertension”. Similar to the Guyton-Coleman model1, this new model correctly predicts the pressure-natriuresis “relationship” and haemodynamic profile of salt-induced hypertension without making assumptions about the primacy of the kidney in hypertension. As with any new hypothesis that directly challenges one that is historical, it is controversial and awaits validation. However, it is this new hypothesis that has initiated our review and that will be discussed.
Hypertension and the need to reduce sympathetic nerve signalling
It is now well accepted that hypertension is associated with chronic activation of the sympathetic nervous system3,4. Several of the recent clinical trials of baroreflex stimulation and renal denervation support targeting the sympathetic nervous system for treatment of chronic hypertension, as well as identifying both the sources and targets of autonomic imbalance. The significance of the new sympathetic hypothesis of hypertension model2 is that emerging therapies designed to target specific sympathetic pathways to treat hypertension may need to extend beyond neural control of the kidney exclusively.
The sympathetic hypothesis for hypertension has parallel, linked afferent-to-efferent paths. On the one hand there is aberrant afferent signalling from visceral receptors to central nervous sensory structures such as the dorsal horn and the nucleus tractus solitarii of the dorsomedial medulla that both radiate to major autonomic controlling regions including the ventrolateral medulla and the hypothalamus. On the other hand, this afferent activity reflexly generates excessive efferent signalling to specific target organs resulting in blood pressure elevation, end-organ disease remodelling and disease. In either case, the vision of selectively modifying either the aberrant afferent signalling into the brainstem or reducing excessive signalling to an end organ independently or together can result in blood pressure improvement. This vision offers the potential to target specific sites within the body selectively to cause restoration of normal pressure without the inherent complications associated with lifelong systemic pharmacologic blockade of the adrenergic system and the often intolerable side effects. It has to be an immediate aim of medicine to provide effective antihypertensive treatment that does not turn an asymptomatic condition into a symptomatic pharmacologically induced “headache”.
The central state of the autonomic nervous system is dependent, in part, upon peripheral sympathoexcitatory and sympathoinhibitory inputs from chemo- and mechanoreceptors, respectively. Such inputs include the carotid body chemoreceptors and arterial baroreceptors. Moreover, it is known that these afferent inputs converge producing occlusive interactions5,6. Pharmacological approaches to lower adrenergic activity are not targeted to individual vascular beds and produce a global depression of sympathetic activity that is effective in lowering blood pressure but also lowers sympathetic activity to non-cardiovascular targets inappropriately. It is this that causes the significant side effects incompatible with a good quality of life. Some drug therapy such as diuretics can increase sympathetic efferent traffic compounding antihypertensive benefit from additional medications and advance target organ damage. Differently, a medical device strategy can alter the central sympathetic state by selectively increasing or decreasing signals from end organs to the autonomic nervous system. Recently, electrical stimulation of the carotid sinus baroreflex or denervation of the renal nerves have served as examples where a single peripheral target has resulted in changes in markers of the systemic sympathetic state. This strategy, selectively modifying the input from a peripheral sensor to the autonomic nervous system, has resulted in dramatic biologic benefits without the myriad of adverse events typically associated with systemic adrenergic pharmacologic blockade. The question prompted is why an interventional strategy may spare side effects and be different from a pharmacological approach aimed at lowering sympathetic activity. An explanation is that an interventional approach permits targeted therapy allowing physiological lowering of sympathetic activity to distinct vascular beds characterised by their functional role in generating vascular resistance. For example, baroreceptors cause sympathoinhibition in skeletal muscle but not cutaneous vascular beds. Thus, such an interventional approach benefits from harnessing the intrinsic physiology and the inherent differential control over the sympathetic nervous system.
Targeted sympathetic ablation
There are probably several distinct peripheral sources of reflexly triggered excessive sympathetic signalling originating from aberrant sensory activity whose selective denervation might restore autonomic balance. Reciprocally, there are probably several peripheral targets where denervation of the efferent sympathetic signals might reduce blood pressure or prevent end-organ disease associated with hypertension. It is probable that the syndrome of hypertension includes several targets that might give optimal results when used singularly or in combination. The concept of “sympathetic signatures” demonstrates the different organ sympathetic activity in different forms of hypertension7. Thus, selecting which interventional approach is going to be most effective will become crucial and will require careful pre-screening of patients. Determining criteria for selection is a topic that currently requires research activity and energy.
An advantage of an interventional approach is that it will permit specific combinations of afferent and efferent denervation strategies, potentially different among patients and customised to the cause(s) of hypertension, which might result in optimal blood pressure and fluid outcomes. Alternatively, the sympathetic signalling from the autonomic nervous system to a single organ target can be modified, resulting in the selective modification of that organ’s sympathetic state, without the complications of systemic adrenergic interference. In this manuscript, we will introduce baroreflex stimulation, renal nerve ablation, splanchnic nerve ablation, and carotid body ablation as four contemporary examples which may one day be used individually or in combination to address chronic diseases and prevent end-organ damage.
BAROREFLEX STIMULATION
Thrasher8 should take the credit for reintroducing interest in the long-term regulation of arterial pressure by carotid sinus baroreceptors. Until his elegant series of studies it was assumed that baroreceptors effectively reset and operate over a beat to beat time scale to buffer acute changes in arterial pressure. Indeed, without their presence arterial pressure showed tremendous lability. By removal of all baroreceptor afferents except for one carotid sinus which was exposed to pressure unloading chronically by stenosis of the common carotid artery, Thrasher showed a well maintained (weeks) hypertensive response. From this has followed the converse experiment electrically to stimulate and reduce arterial pressure in healthy and obese dogs; again the results have been convincing providing evidence of chronic manipulation9.
Carotid sinus baroreflex stimulation has been examined in clinical trials for hypertension. In a remarkable early clinical investigation, Heusser and colleagues10 demonstrated repetitive reduction of muscle sympathetic nerve activity (MSNA) when the device was on and when blood pressure was falling. This confirms that baroreflex stimulation results in reduced peripheral muscle and vessel signalling and is a primary sympathetic mechanism for blood pressure control. It also confirms that reducing MSNA is effective in lowering arterial pressure in patients with hypertension. In the complex Rheos pivotal FDA trial, 322 patients with drug-resistant hypertension underwent implantation with an early-generation carotid sinus leads and stimulator. In this trial, the average blood pressure fall was 35 mmHg at 12 months, with 81% of the patients having a >10 mmHg fall in blood pressure from baseline and over 50% of the recruited patients reaching blood pressure targets at 12 months11. The device has recently undergone dramatic evolution with improvements including a unilateral carotid sinus patch and a smaller implantable device12. Early clinical data in both heart failure with preserved ejection fraction and heart failure with reduced ejection fraction fuel enthusiasm for barostimulation as a treatment for these chronic disorders, with hoped-for improvements both in mortality as well as morbidity and quality of life. Clinical trials in systolic heart failure –the HOPE4HF (NCT01720160), Rheos Diastolic Heart Failure trial (NCT00718939) and Barostim Hypertension Pivotal Trial (NCT01679132)– are actively enrolling.
SELECTIVE RENAL DENERVATION
The reported clinical experience with therapeutic renal denervation demonstrates that reduction of blood pressure is a predictable consequence of selective renal denervation13,14. The mechanism was initially felt to be related to RDN’s direct effect on reducing efferent sympathetic signalling to the kidney confirmed with reduced renal-specific norepinephrine spillover14 with reduction of renin release, and leftward shifting of the pressure-natriuresis relationship. These findings are consistent with the classic Guyton-Coleman model of hypertension1. However, the mechanism may be related to reduction of the kidney’s contribution to central sympathetic tone via afferent renal nerves. The role of renal afferents in hypertension was suggested by documented reduced hypothalamic activity after dorsal rhizotomy in reduced renal mass rats15, and reduction of muscle sympathetic nerve activity following nephrectomy in patients maintained on dialysis16. Renal denervation results in reduction of MSNA and total body NA spillover17 as well as single-fibre MSNA18, both studies confirming that RDN reduces direct neurologic signalling to the brain. Ironically, afferent reduction would result in reciprocal efferent signal reduction, so the discussion about afferent/efferent may be unresolvable unless reinnervation occurs in which the timing for afferent versus efferent fibres is separable, and that arterial pressure remains reset to lower levels.
Recent reports have indicated that renal denervation is not always successful in reducing arterial pressure in all patients (e.g., Brinkmann et al, Chan et al, Prochnau et al, Vase et al.19-22). Here, we will limit our discussion to the study by Brinkmann and colleagues19. In this study, Brinkmann et al19 have presented data on MSNA changes in 12 patients with difficult to treat blood pressure, including four with systolic blood pressure <140 mmHg at recruitment. Their results confirm that renal denervation does not reduce blood pressure in those with near normal pressures, and does not reduce multiunit MSNA in a population with mildly elevated MSNA. The limitations of the small observational series were noted by Mahfoud and colleagues23. Differently, Hering and colleagues18 in a larger case-controlled series of patients, all with confirmed multi-drug-resistant hypertension and substantially elevated blood pressure, reported a mild reduction of multiunit MSNA and significant reduction of single-fibre MSNA. The series differ in both entry blood pressure and multiunit MSNA bursts/100 heart beats, and underlying pharmaceutical use as well as available control populations. The important distinction between multiunit and single-fibre MSNA recordings remains to be elucidated but gives potential mechanistic insight into how sympathetic drive is reduced.
Thus, selective renal denervation is associated with significant, sustained reduction of blood pressure and may derive its benefit through reduction of afferent or efferent signalling, or probably both. Given that renal denervation does not abrogate the need for antihypertensive medication and that recent studies indicate success rates of ~50%19-22, which is substantially lower than those originally found (84%14), this fully justifies exploring additional targets.
TARGETED SPLANCHNIC DENERVATION
Surgical therapy for hypertension in the 1940s and 1950s was particularly successful when the splanchnic region was targeted24,25. However, by the 1960s the advent of safe and effective drug therapies (including ganglionic blockade and guanethidine) led to the abandonment of surgical sympathectomy for treating hypertension.
A recent study compared the effects of renal and splanchnic nerve ablation on arterial pressure and fluid balance in Dahl salt-sensitive (DS) hypertensive rats26. Similar to a significant fraction of humans with essential hypertension, the DS rat is salt-sensitive, has increased renal and splanchnic vascular resistance27,28, and exhibits hypertension-related renal injury29.
Ablation of either the renal nerves (surgical with phenol) or the splanchnic nerves (coeliac ganglionectomy) chronically decreased arterial pressure to a similar magnitude (Figure 1). Interestingly, a combination of the two procedures resulted in an additive effect on arterial pressure suggesting the underlying mechanisms were different (Figure 1).
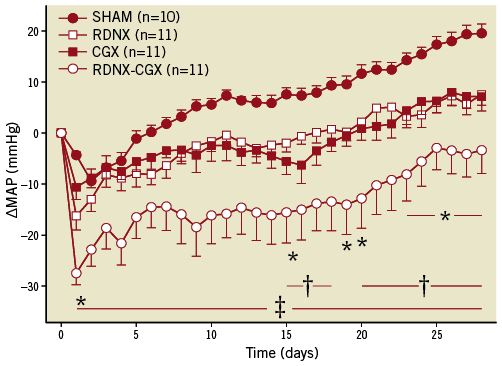
Figure 1. Response of mean arterial pressure (MAP): Sham, renal denervation (RDNX), celiac ganglionectomy (CGX) or combined RDNX-CGX in Dahl S rats on a high salt diet. Results are plotted as the change in MAP (∆MAP) from the day before targeted sympathetic ablation. MAP was ~140 mmHg in all groups prior to the intervention. MAP continued to increase in all groups since the high salt diet was maintained. P<0.05 for RDNX vs. SHAM; †p<0.05 for CGX vs. SHAM; ‡p<0.05 for RDNX-CGX vs. SHAM. Data replotted from Foss et al26.
The mechanism of the antihypertensive response to splanchnic nerve ablation remains to be determined but probably involves both resistance and capacitance vessels in the splanchnic vascular bed. Splanchnic veins are heavily innervated by sympathetic nerves and account for a large fraction of the venous storage capacity of the circulation30. Similarly, since the splanchnic vascular bed receives ~30% of the total cardiac output, neural control of splanchnic arterioles has a major impact on arterial pressure. Ablation of splanchnic sympathetic nerves, by decreasing splanchnic vascular resistance and increasing vascular capacitance, would promote a redistribution of blood from the arterial to venous compartment decreasing arterial pressure. This hypothesis is in line with the new mathematical model of salt-sensitive hypertension in which the distribution of blood volume between a high compliant (i.e., splanchnic) and low compliant (i.e., kidney) vascular bed is determined by neural input to each vascular bed2.
Other studies have shown that hypertension induced by chronic administration of angiotensin II, in combination with a high salt diet, is neurogenically driven and dependent on splanchnic, but not renal, sympathetic nerves31,32. In addition, splanchnic denervation reverses the neurogenically mediated decrease in total vascular capacitance in this model33.
Taken together, reports from early surgical sympathectomy studies in hypertensive humans24,25, recent reports from animal models of neurogenic hypertension26,34, and a new mathematical model of salt-induced hypertension1, all support the concept that targeted ablation of splanchnic sympathetic nerves should be considered as a stand-alone or adjunct antihypertensive therapy.
We next consider a novel approach that may be equally or more effective, as it intervenes with mechanisms that might be, in part, responsible for the aetiology of the heightened vasomotor sympathetic activity in hypertension.
CAROTID BODY ABLATION
It remains enigmatic as to why sympathetic activity rises in conditions of hypertension, but this has to remain a high priority research question. There is no doubt that multiple factors contribute including renin-angiotensin II aldosterone overdrive, organ hypoperfusion, endothelial dysfunction and inflammation4,35. Asking what comes first may be a naïve question as there may be a relative coincidence of prohypertensive factors. Further, delineating cause from effect is notoriously difficult in a fully integrated system. However, an emerging concept is that excessive sympathetic signalling is a product of high sensory drive from cardiorespiratory sensors. It may not be that those that simply “scream” loudest generate the highest sympathetic activity; rather, those that are most critical in the survival of an animal with direct, powerful excitatory connections to oscillators generating sympathetic activity could make potent, antihypertensive targets; the carotid body may be one of them.
With their strategic location at the root of a major source of blood flow into the brain and their powerful activation of the sympathetic nervous system, the carotid bodies are a potential target. The carotid body resides at the bifurcation of the common carotid artery and is innervated by a branch of the glossopharyngeal nerve called the carotid sinus nerve. It also receives innervation from the cervical sympathetic ganglion. It has the highest blood flow per gram of tissue of any organ in the body (2000 mL/min per 100 mg of tissue). In the spontaneously hypertensive (SH) rat it is hypertrophied suggesting upregulation of protein synthesis and there is angiogenesis within the microvasculature relative to adult controls36. As previously stated by Ponte and Purves37: “their function of ensuring adequate oxygenation of the brain may be the most important one they possess”. This must include maintenance of cerebral perfusion brought about by increasing arterial pressure. With direct input to the caudal, commissural nucleus tractus solitarii, the carotid body input provides excitatory synaptic drive to neurons that is both powerful and long-lasting relative to other inputs5. Carotid chemoreceptors generate sympathetic activity directly by activating rostral ventrolateral medullary presympathetic neurons38 and indirectly by engaging respiratory neurons connected to these sympathetic neurons39. Importantly, carotid chemoreceptors are essential for inducing chronic potentiation of sympathetic activity and hypertension seen after intermittent hypoxia in rats, for example40. Thus, their activity can cause long-term synaptic plasticity within brainstem sympathoexcitatory networks. Further, it is well known that in hypertension (but also in heart failure) there is an increase in carotid body reflex sensitivity such that evoked increases in sympathetic activity are larger in hypertensive animals41 and humans42,43 and that this occurs before the onset of hypertension41. It is also known that, in addition to elevated reflex sensitivity, carotid bodies in spontaneously hypertensive (SH) rats44 and human hypertensive patients45 also possess aberrant tonicity. The cause for this pathological tone is not known.
Given this information, it might be predicted that severance of carotid body afferent input in SH rats would reduce arterial pressure. Given that the reflex sensitivity was already heightened in juvenile prehypertensive rats41, Abdala et al44 have shown that bilateral denervation of the carotid sinus nerves significantly reduced the development of hypertension (Figure 2). In adult SH rats the procedure gave a prompt, robust fall in arterial pressure (Figure 3A), which was maintained for weeks. The antihypertensive effect was associated with a reduction of sympathetic vasomotor tone, as determined by reductions in low-frequency spectra of systolic pressure (Figure 3B) and reduced falls in arterial pressure after ganglionic blockade (Figure 3C)44. There was also a transient reduction in respiratory frequency (Figure 3D). Surprisingly, baroreceptor reflex sensitivity was improved, which must reflect gain of function from the aortic arch baroreceptors reflecting the antagonistic central action between these afferent systems mentioned above5,6,46.
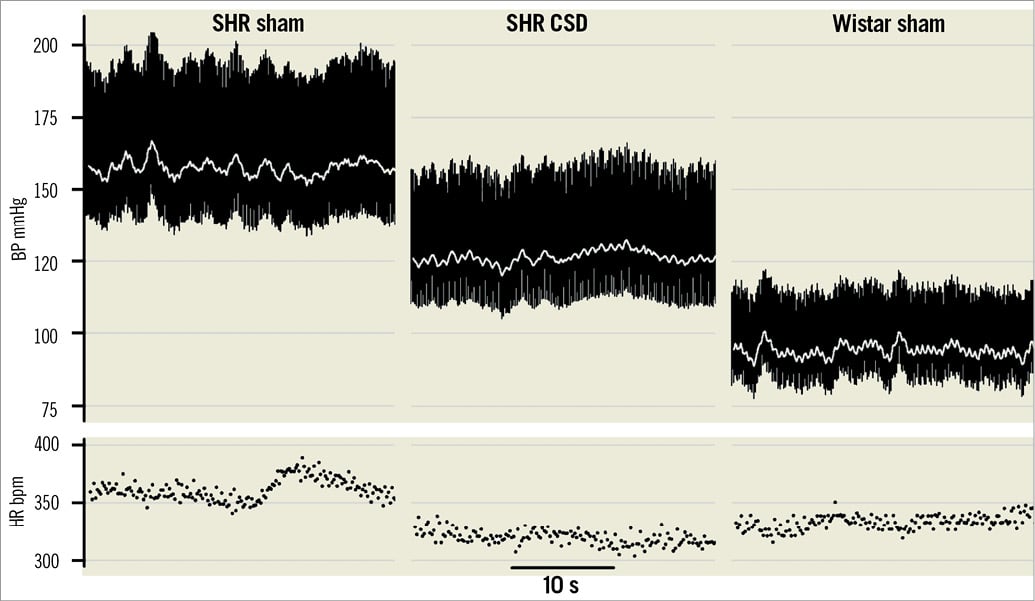
Figure 2. Carotid body is crucial for the development of hypertension in the SH rat. Pulsatile arterial blood pressure measured in three rats: two sham operated (SH and Wistar) and in a SH rat after carotid sinus denervation (CSD). All operations were performed around 4 weeks of age before SH rats develop hypertension. Note that in the absence of carotid chemoreceptor input the hypertension developed is less in the SH rat. The change in heart rate was not significant between sham and CSD SH rats. Data modified from Abdala et al44.
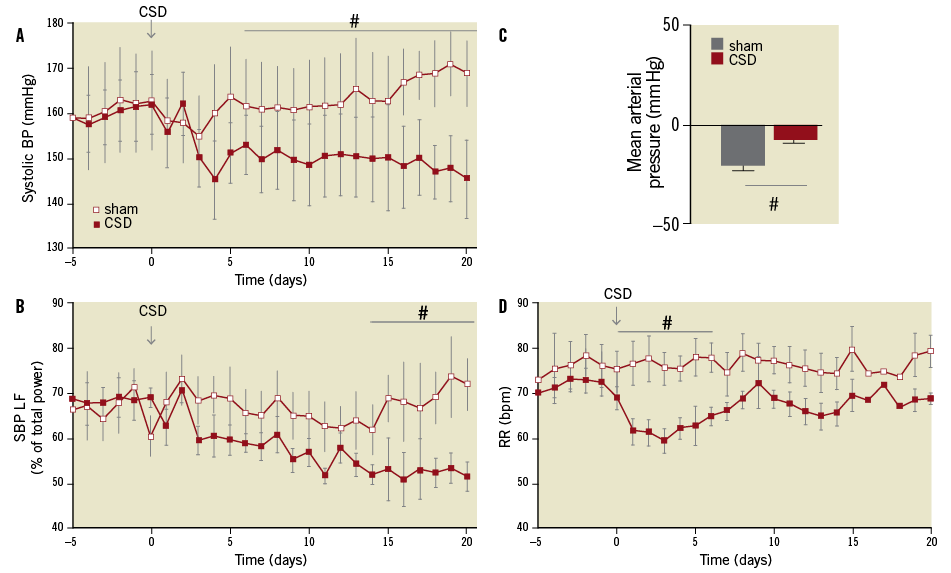
Figure 3. Carotid sinus denervation (CSD) reduces arterial pressure and sympathetic tone in adult SH but not age and sex matched Wistar rats. In the adult SH rat there was a prompt, substantial and long-lasting fall in systolic arterial pressure (A). Note the age-dependent increase in arterial pressure (A). The fall in systolic arterial pressure was associated with a reduction in the low-frequency spectra indicative of a reduction in sympathetic vasomotor tone (B). C shows that the depressor effect in response to ganglionic blockade (hexamethonium, 10 mg.kg-1 IV) after CSD was smaller than that seen in sham operated SH rats supporting less vasomotor sympathetic tone. CSD produced a transient fall in respiratory frequency (D). Data modified from Abdala et al44.
The issue of whether carotid body ablation is a safe procedure in humans is supported by earlier studies in which carotid bodies were resected either unilaterally or bilaterally to alleviate dyspnoea in patients with asthma and chronic obstructive pulmonary disease (reviewed in Paton et al47). This effect may be mediated by loss of the sensory perception of dyspnoea and/or improvement in airway resistance consequent on re-establishing autonomic balance in the nerves regulating bronchomotor tone. In published studies there were no significant adverse events that were related to a reduction in carotid body afferent input (reviewed in Paton et al47). A total absence of carotid body chemosensory drive might bring into question the effect on sleep-disordered breathing, a condition commonly associated with hypertension48. Given the overwhelming evidence for the involvement of carotid bodies in the initiation of sleep-disordered breathing49, it is hypothesised that removal of this afferent will have a positive outcome on sleep apnoea.
An attraction of ablating a prohypertensive afferent system is that it will affect numerous vascular beds controlling vascular resistance simultaneously providing a robust response but probably spare those sympathetic outflows to non-cardiovascular targets (Figure 4). A robust response may allow reduction in drug doses and even withdrawal of medications. Whether it proves successful in humans awaits the test of time but a previous report has shown encouraging data. Nakayama and colleagues50 reported that bilateral carotid body denervation lowered blood pressure in hypertensives, was without effect in normotensive patients, and caused a rise in hypotensive patients to near normal; these effects persisted throughout the six-month follow-up period. Given these positive preclinical outcomes, it is justifiable to assess the potential of therapeutic carotid body modulation.
Combined interventional therapy
Preclinical data support the possibility of combination interventional therapy (Figure 4). As with combined renal and splanchnic nerve denervation (Figure 1), combining carotid sinus nerve ablation with renal denervation in SH rats provided a summative response (FD McBryde, AP Abdala and JFR Paton –unpublished data) indicating separate mechanisms. This supports the idea that either splanchnic nerve denervation or carotid body ablation after renal denervation would be an effective strategy in human hypertensive patients especially those who remained hypertensive, did not show a substantial blood pressure lowering response or were drug-intolerant. We are unaware of any preclinical data reporting the type of interaction between baroreflex stimulation and renal denervation, coeliac ganglionectomy or carotid body ablation. This is an area of science that needs attention.
Conclusion
Hypertension, historically viewed as a syndrome of disorders inherently requiring renal participation, appears more broadly to include a substantial contribution of autonomic imbalances. Excess peripheral chemoreceptor signalling, or impaired mechanoreceptor signalling, results in autonomic imbalance, logically addressed by the selective ablation of sources of excessive signalling or stimulation of the carotid sinus baroreceptors (Figure 4). Additionally, organ-selective sympathectomy can result in reduced blood pressure and perhaps end-organ preservation. This idea, selective stimulation or ablation of sources of afferent and efferent sympathetic signalling, leads to a vision of interventionalists targeting specific sites to restore autonomic balance, thereby providing a novel therapy for the many diseases linked together by chronic autonomic imbalance (Figure 4). Five years ago, a specialist hypertension physician was struggling to find a new strategy to control hypertension in refractory patients. Now the physician may be faced with an opposite problem, namely which interventional approach to adopt. It therefore seems imperative that, given the irreversibility of many of these procedures, criteria are needed which assist in selecting patients into the best treatment route that will give optimal antihypertensive benefit and relieve patients of drugs with severe side effects. This presents us with a new and significant research challenge for the future.
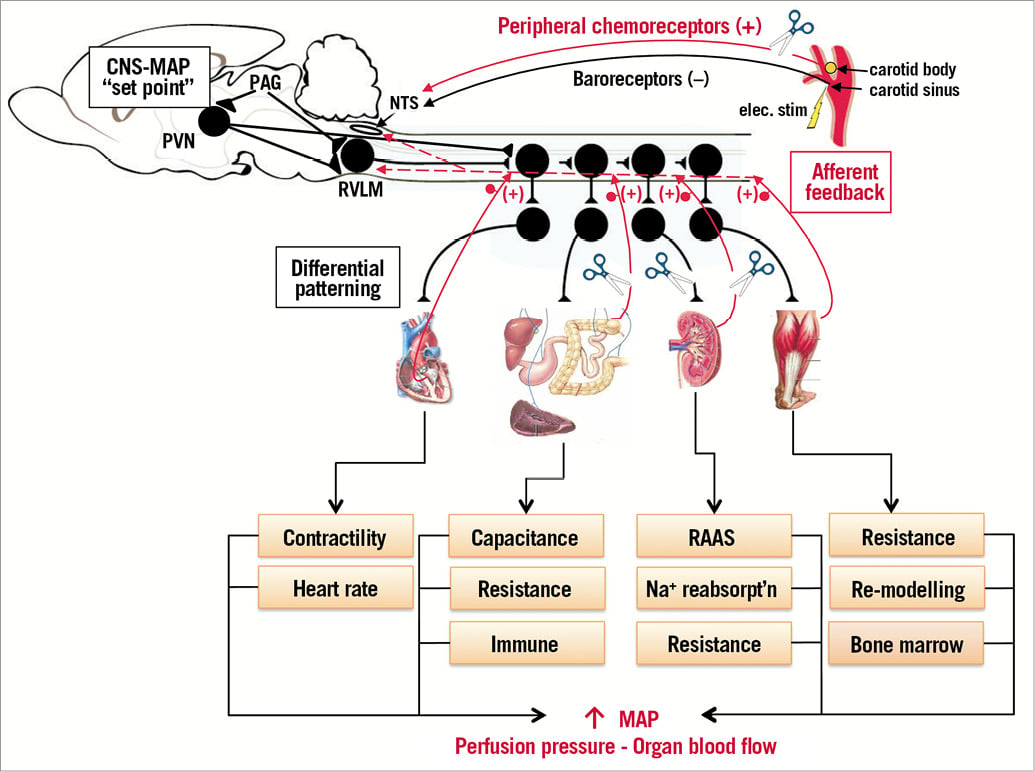
Figure 4. Sympathetic nervous system control and interventional approaches to control it in conditions of drug-resistant hypertension. Sympathetic activity set-point remains undefined but is controlled from areas of the central nervous system including the nucleus tractus solitarius (NTS), hypothalamic paraventricular nucleus (PVN), rostral ventrolateral medulla (RVLM), periaqueductal grey (PAG) and spinal circuits. We have reviewed preclinical and clinical data showing how electrical stimulation (elec. stim) of the carotid sinus, renal afferent/efferent denervation, targeted splanchnic denervation and carotid body ablation can all modulate sympathetic nerve activity/arterial pressure chronically thereby providing much needed non-drug-related clinical approaches for the treatment of refractory hypertension. We wish to emphasise a role for the PAG in controlling human hypertension51 and that sympathetic discharge is patterned differentially. Note that sympathetic activity controls arteriole resistance, venous capacitance, can cause vascular hypertrophy, activates the renin-angiotensin-aldosterone system (RAAS), drives renal function (e.g., sodium reabsorption) and is pro-inflammatory causing T cell activation. In turn, these all feed back reciprocally to excite neuronal networks generating sympathetic activity4. Each end organ depicted (heart, gastrointestinal, kidney, skeletal muscle) can feed back to raise sympathetic activity through spinal and supraspinal reflex circuits. Our hypothesis is that aberrant afferent feedback can lead to neurogenic hypertension. (+): sympathoexcitatory; (–): sympathoinhibitory
Conflict of interest statement
P. Sobotka is an employee of Cibiem, Inc. and receives royalties based on sales of the Medtronic-Ardian Symplicity renal denervation system., and is a consultant to Medtronic Inc., Rox Inc. and Ardelyx, Inc. J. Paton is a consultant to Cibiem Inc. J. Osborn is a consultant to Medtronic Inc.

