Abstract
Aims: Device-based arterial closure is currently used to achieve haemostasis following percutaneous intervention. Little is known about the in vivo patterns of device absorption. We aimed to characterise the absorption dynamics following implantation of the Angio-Seal™ VIP closure device (AVCD) (St. Jude Medical, St. Paul, MN, USA) by using serial intravascular ultrasound (IVUS) and histology.
Methods and results: Eleven AVCD were implanted following 6 Fr femoral arterial sheath in six pigs. Using carotid access, angiograms and IVUS were performed at baseline, 3, 5, 7, 14, 30 and 42 days following deployment. At termination, arteries were processed for histology at 14 (n=3), 30(n=4) and 42 days (n=4). By IVUS, following implantation the intravascular component (IC) area remained unchanged up to 14 days and decreased by 50% at 30 days and 95% by 42 days. By histology, there was a progressive decline in the IC area at 14 days and decrease by 30% at 30 days and 77% by 42 days. Histology demonstrated almost complete absorption of the IC and no signs of severe chronic granulomatous inflammation.
Conclusions: IVUS serial imaging demonstrated almost complete absorption of the IC by 42 days in normal porcine femoral arteries. There was no evidence of severe chronic granulomatous vascular inflammation demonstrated by histology.
Introduction
The Angio-Seal™ vascular closure device (AVCD) is designed to close the arteriotomy site following percutaneous coronary intervention and consists of an intravascular anchor (dimensions 10 mm x 2 mm x 1 mm) and a small extravascular bovine collagen component that are maintained together using a suture mechanism.1 Following device deployment, vessel haemostasis is achieved by controlled compression of the vessel wall that occurs between both components. The intravascular component of the device, designed to anchor the device to the inner vessel wall, is composed of a bioabsorbable mixture of polyglycolide and polylactide polymers.2,3 In the clinical setting, clinical studies have been published in regards to the safety, efficacy and clinical applicability of this device.4-6 However, most of these reports focus on clinical outcomes among all device technologies and little on biological effects of the device on the vessel wall.7-9 In this study we aimed to analyse the patterns of absorption and vascular healing of the AVCD in an in vivo environment by performing intravascular ultrasound (IVUS) at different time points after device deployment in normal porcine femoral arteries.
Methods
Experimental design
The study was approved by the Institutional Animal Care and Use Committee. All animals received standard care pursuant to human cathlabs protocol following the act of animal welfare and the “Principles of Care of Laboratory Animals” formulated by the Institute of Laboratory Animal Resources (National Research Council, NIH Publication No. 85-23, revised 1996). A total of six domestic Yorkshire pigs weighing more than 50 kgs were used in this experiment. One intramuscular (IM) muscarinic anticholeinergic dose (glycopyrrolate, 0.2 mg/ml, dosage 0.005-0.02 mg/kg) was given preprocedure. Induction was achieved with a rapid acting general anaesthetic (tiletamine + zolazepam, telazol™ 100 mg/ml, dosage 2-5 mg/kg). Animals underwent endotracheal intubation and maintained with 1-3% of continuous inhalation of isoflurane. Under general anaesthesia percutaneous access was obtained in both common femoral arteries using Seldinger technique and bilateral 6 Fr sheaths were introduced. Carotid access was also established and anticoagulation with heparin was achieved (3,000-10,000 U maintaining a coagulation time ≥250 seconds). Vascular closure devices (6 Fr) were placed (Angio-Seal™ VIP devices, St Jude Medical, St. Paul, MN, USA) in each femoral artery using regular clinically indicated technique. One device was misplaced and was excluded from the study. Via carotid access, standard angiography and IVUS were performed after device implantation – and in a series of predetermined time points – in order to measure the degree of device absorption. The isoflurane was discontinued and the animals extubated when the gag reflex had returned. Buprenorphine 0.01-0.02 mg/kg IM and flunixin 1-2 mg/kg IV was injected for routine pain management. Animals received cefazolin to prevent infections (1 g IV). The follow-up times were chosen to assess the acute, subacute and chronic patterns of device absorption (Table 1). At the designated endpoint, the animals were euthanised while under anaesthesia by IV injection of pentobarbital euthanasia solution (100 mg/kg) and/or potassium (40 mEq).

Angiography and quantitative analysis
The 6 Fr guiding catheter was advanced under fluoroscopic guidance through a guidewire using carotid access. At baseline, initial identification of the common femoral arteries was performed using angiography. The region of interest, defined as the site at which the device was placed, was identified and labelled. Based on previous baseline angiography, nitroglycerin (100-200 µg) was administered intra-arterially during the intervention as needed and post angiography in the same dosage to prevent or relieve vasospasm. The area occupied by the intravascular component, seen as a hypo-dense structure on angiography, was assessed using Quantitative Analysis (QA) at each time point. QA was performed by a single analyst blinded to treatment and time point. Quantitative analysis software package version 4.0.12 of GE Medical Systems (GE; GE Healthcare Ltd, Little Chalfont, Buckinghamshire, United Kingdom) was used throughout the study. This proprietary software utilises known catheter size for calibration.
Intravascular ultrasound imaging protocol
IVUS pullback images were obtained and analysed using a peripheral ultrasound catheter (Atlantis® SR Pro 40 MHz Coronary Imaging Catheter, Boston Scientific, Natick, MA, USA) and a commercially available measurement analytic system (iLab; Boston Scientific, Natick, MA, USA). Using fluoroscopy, the IVUS catheter was placed distal to the site at which the device was deployed, and an automated pullback performed at a speed of 1 mm/sec covering 10 mm proximal and distal to the implantation site. The starting position of the IVUS catheter was determined by fluoroscopy and situated by anatomical landmarks in live image during the pullback. The automated pullbacks were gated to the electrocardiogram to enable reconstruction of longitudinal view of the vessel. This analysis was developed to enable measurements of both the cross-sectional and longitudinal diameters of the intravascular component of the device. Two automated pullbacks of each device were recorded under the same study time point, and the image with the best quality was selected for analysis. The morphometric analysis of the vessel was performed using standard definitions previously published.10 IVUS analysis was performed by two operators blinded to the follow-up time points. Each individual pullback analysis was divided into three separate vascular segments: normal proximal reference (10 mm before the intravascular component), intravascular component segment and normal distal reference (10 mm after the intravascular component). The analysis included lumen area and diameter, as well as arterial wall thickness in the three studied vascular segments. In the intravascular component segment, the cross-sectional diameter (thickness) and length were also measured. The area of the intravascular component was determined in cross-section images of IVUS after implantation and through all the follow-ups (3, 5, 7 days) and termination points (14, 30, 42 days). In addition, arterial wall thickness was also measured in order to evaluate the dynamics of vascular healing.
Histology processing protocol
After completing the last follow-up, all animals were sacrificed after angiographic and IVUS evaluation. Dissection of the vascular tree was performed down from the lower abdominal aorta to the popliteal artery. After harvesting the femoral arteries, the extracted arterial segment was perfused with one litre of normal saline at 100 mmHg of pressure followed by pressure perfusion of 10% neutral buffered formalin for a total of 24 hours. The femoral arteries were extracted from the vascular tree 10 mm proximal and distal from the device. The femoral arteries were further harvested into 2-3 mm arterial segments from proximal to distal. Arterial segments for histology were analysed following the same vascular areas analysed in IVUS (proximal and distal normal references and intravascular component vascular segment). Following anatomical references, vascular orientation was maintained along the histological process. Once in paraffin blocks, 5-µm sections were obtained and stained with hematoxilyn and eosin and Movat Pentachrome. (Table 1)
Statistical analysis
The results from the IVUS analysis at sacrifice were compared to histology morphometric analysis. All time points within IVUS pullbacks were also analysed between them. Averages and standard deviations were calculated for each measurement in each endpoint group to obtain parametric data. The statistical analysis was performed using SigmaStat 3.11 software (2004, Systat Inc. San Jose, CA, USA). A P value of 0.05 or below was considered of statistical significance.
Results
Angiographic quantitative analysis
The mean vessel diameter at which all AVCD were implanted was 4.8±0.41 mm. The intravascular component decreased its size by 7% after the first week follow-up measured by quantitative angiography. After day 14, the intravascular component performed greater absorption, it reduced its size by 67%. There was a reduction of 65% between day 14 and 30 seen in angiography. After day 30, plain angiography was unable to detect the presence of any intra-vascular structure (Figure 1). Angiographic evaluation demonstrated no signs of blood flow impairment or evidence of residual vessel stenosis at the implantation site at any time.
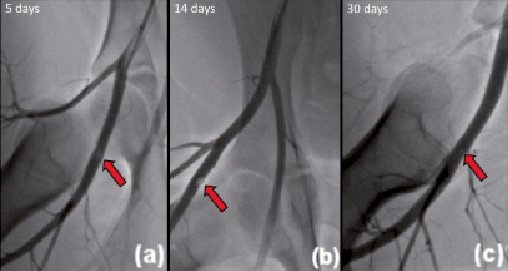
Figure 1. Representative angiograms at different follow-up time points. Angiography shows the intravascular component (a) at five days and (b) at 14 days (arrow). The device is no longer visible at 30 days (c) following implantation. No impairment in blood flow was observed at any follow-up time point.
IVUS analysis of absorption dynamics
The intravascular component maintained its dimensions with no statistical difference between the first week after implantation and the 14-day follow-up by IVUS (p < 0.21). Between day 14 and 30 there was a 50% reduction in the area of the intravascular component. From this point on there was a progressive decline in the size of the area of the intravascular component, with an additional 45% decrease in size by 42 days (p <0.001; Figure 2).
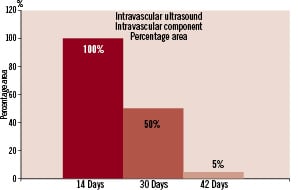
Figure 2. Cross-sectional in vivo evaluation of the bioabsorption patterns of the intravascular component of AVCD during different time points by IVUS analysis.
The cross-sectional diameter of the device measured by IVUS followed a similar pattern of absorption. Longitudinal IVUS analysis showed that the mean length of the intravascular component was 9.5±0.6mm at the time of implantation. The length of the device decreased by 34% by seven days. The length of the device remained stable up to 30 days and dramatically decreased by 58% in length by 42 days (p <0.001, Figure 3).
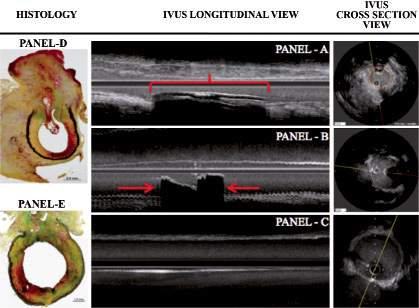
Figure 3. Representative IVUS and histological images of the AVCD intravascular component. At post implantation, IVUS shows the intravascular component (red bracket) displaying well-defined echogenic borders producing a shadow (Panel A). At seven days (Panel B), the length of the intravascular component has considerably reduced in size (approximately 50%). At 42 days (Panel C), the intravascular component is not identifiable in the longitudinal view and barely seen in the cross section view. Panel D shows a representative histological image at 30 days. Panel E demonstrates almost complete absorption of the intravascular component by histology.
Histology-IVUS correlation
The bioabsorption observed in the intravascular component via IVUS correlated very similarly to the histological analysis performed at 14 and 30 days (Figure 4).
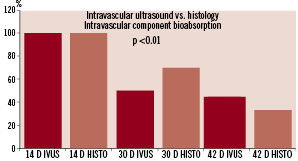
Figure 4. Comparison of the intravascular component area using IVUS and histological analysis.
Histological evaluation of the intravascular anchor demonstrated a progressive absorption pattern that started at 14 days and progressively decreased 30% at 30 days and 77% by 42 days following implantation (P <0.01). At 42 days, the intravascular component is almost completely absorbed and its remainder appears to be completely embedded into the vessel wall. A similar pattern of slight lumen reduction following device implantation was confirmed by histological evaluation. Also, there was an early increase in the total vessel wall area causing a transient pattern of positive remodelling at the implantation site. The intimal/media ratio at the implantation site was comparable to the normal reference segments at 42 days. At 14 days, there was minimal degree of mononuclear cell infiltration surrounding the extra-vascular component that progressively decreases over-time and is no longer apparent by 42 days. At 42 days, there was almost complete absorption of the intravascular component without any evidence
of vascular remodelling, granulomatous or foreign body reaction.
Discussion
The AVCD’s design is unique as it involves an intravascular anchoring mechanism connected to an extravascular anchoring component allowing fixation on the arteriotomy site using a bioabsorbable suture mechanism. This particular device has been in clinical use for over a decade, and has evolved through several device generations. The safety and efficacy of this device11 has been reportedly demonstrated in several clinical trials comparing this technology to other VCDs and manual compression.12-19
Reports derived from clinical registries have shown that the rate of absorption of the intravascular anchor of the AVCD occurs between 60 and 90 days following device implantation.20-24 However, there is little data regarding the in vivo dynamics of device absorption and vascular remodelling occurring after device implantation over different time points. In our study, we deployed devices in arterial segments similar to the human femoral artery in terms of size and location. Our major finding was that following device implantation, the size of the intravascular component remains stable during the first two weeks, then through rapid degradation achieves almost complete absorption by 42 days following implantation. Interestingly, although angiography detected the intraluminal component right after implantation, the intraluminal component was practically “invisible” to this imaging technique two weeks post-device implantation, despite IVUS evidence to the contrary.
IVUS analysis demonstrated that immediately following device implantation there was a slight decrease in the total vessel lumen area, which remained unchanged during the first two weeks following device implantation. After that initial two-week period, the lumen areas begin rapidly increasing reaching normal values between day 30 and 42. Although the total vessel wall area appears unchanged during all follow-up time points, the true value of this parameter is questionable as it was challenging to measure due to the echogenicity of the device that frequently produced a shadow over the vessel wall borders. The in vivo patterns of absorption of the intravascular anchor demonstrated that, following device implantation, absorption appears to occur more rapidly in the longitudinal axis of the device. By seven days post-deployment, the total length of the device decreases by more than 50%. However, the total cross-sectional area starts to decrease significantly two weeks following device deployment. One important limitation of our study is related to the enhanced echogenicity of the intravascular component of the AVCD which produces an acoustic shadow partially “covering” the arterial wall. Therefore, although an overall size of the component was always achieved, it is possible that all resulting dimensions could have been overestimated due to the nature of the artifact. However, we attempted to correct this phenomenon by measuring the intravascular component area, instead of fixed assumed diameters by using a standard methodology in every single time point.
The in vivo evaluation of device absorption correlated with the histological findings. The presence of the intravascular anchor was confirmed to be intraluminal during the first two weeks after implant. In addition, shortly after device deployment there is a rapid increase of the total vessel area causing a compensatory positive vascular remodelling that appears to normalise the slight lumen loss area caused by device implantation. This phenomenon of positive vascular remodelling appears to normalise at two weeks during which both the intravascular component starts rapid absorption and the lumen area begins normalisation. By 42 days post-deployment, all arteries demonstrated complete healing and there was no evidence of residual chronic granulomatous inflammatory reaction or vascular remodelling. It is important to emphasise that the observed absorption characteristics described in this study are the result of the interaction of the device with a non-diseased artery. It is possible that the absorption patterns seen in devices deployed on atherosclerotic arteries may differ, and these differences need to be studied even further.
Previous studies compared Angio-Seal™ with VCDs of different design. Gargiulo NJ et al described the healing characteristics resulting of the implantation of the Angio-Seal™ versus the suture-based Perclose™ VCD deployment.25 In this study, it was observed that the Angio-Seal ™ device resulted in greater peri-adventitial scar thickness compared with the suture based Perclose™ device at 28 days. Important methodological differences exist between this manuscript and our present work. First of all, the main differences between vascular healing patterns in dogs and pigs are not completely clear. In addition, although the extra-vascular material universally seen following device implantation can be interpreted as “excessive scar tissue”, it only represents inert material that is temporarily present and dissolves over time.
Sanghi P et al8 compared the histopathological response to Angio-Seal™ versus Starclose™ deployment in the normal porcine artery. In this particular study it was claimed that at early follow-up (seven and 30 days), Angio-Seal™ arteriotomy closure sites were associated with higher inflammatory scores, but no difference was seen at late (60-day) follow-up. In this study, moderate scores (>101 cells per HFP) were more prevalent in the Angio-Seal™ group within the same 30 days. However, beyond 30 days, the amount of inflammatory infiltrate appears to be more prevalent in the StarClose™ device. It is important to note, that the reported inflammatory score was based on total number of inflammatory cells, but no chronic granulomatous reaction, which was not present in any of the samples studied in our study. Although, there seems to be a difference in terms of the degree of inflammation between these two devices, the differences in follow-up times and scoring methodologies may explain the apparent differences reported by both papers.
However, in general, both papers agree that beyond 30 days, any degree of cellular infiltrate starts to decrease and appears to resolve within 42 and 60 days.
In summary, following device deployment the AVCD induces a slight decrease in lumen area that is rapidly compensated by positive vascular remodelling occurring during the first two weeks. The intra-vascular anchor starts to absorb as early as seven days in the longitudinal axis, and achieves the highest absorption rates between 14 and 30 days. Measured by IVUS, there was evidence of almost complete absorption of the intravascular component by 42 days. However, histology demonstrated that residual components of the device still remain within the vessel wall. Per the Angio-Seal™ Instructions For Use manual, re-entry 1 cm proximal to the previous access site can be performed safely, based on published medical literature.22,26 Based on IVUS and histology data, it seems safe to conclude that re-intervention of the previously closed arterial site could be achieved after two weeks, because the vessel site has regained its baseline normal lumen area and the intravascular anchor has begun rapidly to absorb. The relevance of these findings need to be confirmed in vivo, as the devices were deployed in normal femoral arteries devoid of atherosclerotic tissue that may affect the patterns of device absorption and vascular healing.

