Abstract
Aims: The aim of the pilot SECRITT trial was to evaluate the safety and feasibility of sealing the high risk IVUS and optical coherence tomography-derived thin cap fibroatheroma (TCFA), with a dedicated nitinol self-expanding vShield device.
Methods and results: After screening with angiography, fractional flow reserve (FFR), intravascular ultrasound virtual histology (IVUS-VH) and optical coherence tomography (OCT), 23 patients met enrolment criteria (presence of non-obstructive VH-derived TCFA lesion with thin cap on OCT) and were randomised to vShield (n=13) versus medical therapy (n=10). In the shielded group, baseline percent diameter stenosis was 33.2±13.5%, FFR was 0.93±0.06. At six-month follow-up in shielded patients percent diameter stenosis further decreased to 18.7±16.9% and FFR remained the same 0.93±0.05. Average late loss was 0.24±0.13 mm. Average baseline fibrous cap thickness was 48±12 µm. After shield placement at six-month follow-up neo-cap formation was observed with average cap thickness of 201±168 µm. There were no dissections after shield placement and no plaque ruptures. In addition, mean stent area of 8.76±2.16 mm2 increased to 9.45±2.30 mm2, that is by 9% at six-month follow-up. The number of malapposed struts decreased from 10.7% to 7.6% and the number of uncovered struts at six months was 8.1%. There were no device-related major adverse cardiovascular events (MACE) events at six-month follow-up.
Conclusions: High risk plaque passivation and sealing with a vShield self-expanding nitinol device appears feasible and safe. A long-term larger randomised study with streamlined screening criteria is needed to evaluate the efficacy of this approach over medical therapy.
Abbreviations
TCFA: thin cap fibroatheroma
CSA: cross-sectional area
MI: myocardial infarction
ARC: Academic Research Consortium
ISA: incomplete stent apposition
MACE: major adverse cardiovascular events
IVUS: intravascular ultra-sound
IVUS-VH: intravascular ultrasound virtual histology
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
FFR: fractional flow reserve
Introduction
Our current understanding of the pathogenesis of acute coronary syndrome, the progression of coronary artery disease and sudden death is that 70% of the time patients with atherosclerosis and fatal myocardial infarction incur plaque rupture of the so-called thin cap fibroatheroma and in the rest of the cases pathology reveals plaque erosion or calcified nodule1-3. Many of these plaques have gone undetected by conventional coronary angiography because the underlying lesion was non-obstructive (<50% diameter stenosis) due to the so-called Glagov effect (positive remodelling at the site of large plaque burden). High-risk plaque is defined as a large lipid pool , thin cap (less than 65 µm) and macrophage dense inflammation, as well as positive remodeling2,4-6. The majority of these plaques occur in the proximal portion of the three major epicardial coronary arteries7,8. It is also becoming clear that obstructive plaques (with minimal luminal area <4 mm2) can also be high risk and identify a patient at risk of future events. In fact these plaques have been shown to result in the highest number of events in the PROSPECT trial9, the first prospective natural history study of atherosclerosis using multimodality imaging. Currently there are two strategies for managing patients with thin cap fibroatheromas: 1) Conservative medical therapy based on the premise that none of the imaging modalities to-date have been able to identify reliable features of the plaque that render it prone to major adverse cardiac events, and 2) focal treatment to seal and passivate the plaque. The latter approach has been recently demonstrated in the VELETI trial to prevent progression of disease in vein grafts with non-obstructive lesions10. The SECRITT trial is a randomised, controlled pilot study that evaluates the safety and feasibility of sealing the high risk IVUS and OCT-derived TCFA with a dedicated nitinol self-expanding vShield device. As such, it is the first trial of a dedicated device for treatment of “vulnerable plaque” in native coronary arteries.
Methods
Device description
The vProtect™ luminal shield system (Prescient Medical, Inc., Doylestown, PA, USA) consists of the self-expanding (nitinol) vascular shield (Figure 1A) and a rapid exchange delivery system. The delivery system is compatible with 0.014” guidewires and 6 Fr guiding catheters. The delivery system consists of a distal outer sheath that houses the luminal shield and an inner body with radiopaque markers at the distal and proximal ends of the shield. The luminal shield is constructed from a nickel-titanium alloy with an austenitic finish. The shield has a wall thickness that is less than 70 µm and has been designed with the objective to match the elastic properties of the TCFA. The shield is available in 3.5 mm, 4.0 mm and 4.5 mm diameter with a length of 15 mm for all the diameters. This allows vessels of between 2.75 mm to 4.0 mm to be treated. The distinctive feature of the shield is the hysteresis between the inward radial resistive force and the outward force exerted on the vessel wall. The latter is very low not exceeding 100 mm Hg (Figure 1B) thereby minimising the trauma to the vessel wall and potential for plaque rupture during the deployment.
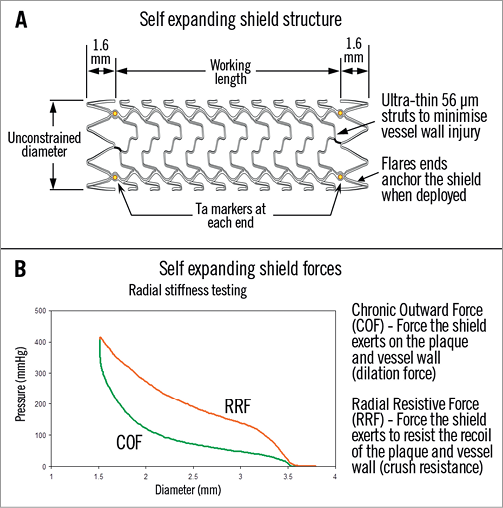
Figure 1. A) Device design and structure highlighting the ultra-thin struts and tantalum markers to allow for positioning. B) Hysteresis curve between radial resistive force and chronic outward force (COF) exerted by the device on the vessel wall. In the case of the vShield, COF is around 100 mmHg, minimising vessel trauma and allowing for gentle continued expansion over time (9% at six months).
Study design and patient population
SECRITT is a clinical prospective pilot, open, single centre randomised study assessing the safety and feasibility of shielding the non-obstructive IVUS-derived TCFA, and the effects on the prevention of plaque progression at six months follow-up. Patients over the age of 18 admitted with stable or unstable coronary syndromes (including non-ST-elevation myocardial infarction) and an angiogram demonstrating the need for PCI in one or more lesion, and concomitant presence of angiographically and haemodynamically non-obstructive IVUS-derived TCFA were eligible for the study. After obtaining informed consent and successful treatment of the culprit lesion (Figure 2) patients were randomised 1:1 to treatment with the shield device or medical therapy. Exclusion criteria were as follows: acute myocardial infarction, prior coronary artery bypass graft (CABG), significant left main disease, cardiogenic shock, renal insufficiency (cr >1.5 mg/dL), resuscitation or intubation, cerebrovascular event within the last 30 days, major bleeding event within the last 30 days, severe hypertension refractory to medical therapy, history of significant trauma or surgery within the last six weeks, know nickel allergy, allergy to aspirin or clopidogrel that cannot be treated, pregnancy, coexisting condition with life expectancy <12 months and vessel diameter on angiography of <2.5 or >4.0 mm. All patients in the study were on aspirin therapy and received clopidogrel loading dose (600 mg) or were on maintenance clopidogrel dose. Anticoagulation during the procedure was achieved with heparin (with goal of ACT >300 msec). After the procedure all patients received aspirin and clopidogrel. All patients were treated with anti-cholesterol medications with the goal of low-density lipoprotein <70 mg/dL. The study protocol was approved by the institutional ethics committee and all patients provided signed informed consent.
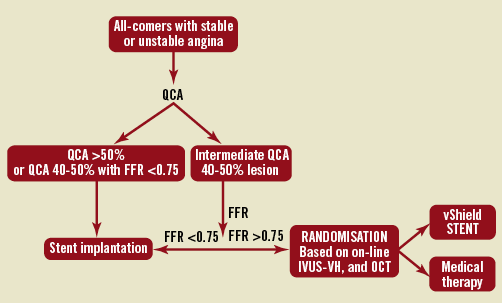
Figure 2. Flow chart.
Study lesion definition
Lesions qualified as study lesions if: 1) they were angiographically intermediate with 40-50% diameter stenosis, and 2) had an FFR of more than 0.75 (pathway B in the flow chart, Figure 2), and 3) fulfilled the criteria for IVUS-derived TCFA. Cap thickness and presence of the lipid pool was also documented by OCT.
Quantitative angiography
The target coronary segment was filmed in two orthogonal planes that had been prescribed after viewing of the preceding angiogram. Quantitative coronary angiography (QCA) was performed following administration of 100-200 micrograms of nitroglycerine to assess the proper length and diameter of the vessel. A final angiogram was made under the same rotation and skew angles following intracoronary nitroglycerine administration. A QCA off-line using CMS-Medis quantitative angiography (Medis, Leiden, The Netherlands) was made to quantify the final result. The following measures were obtained for each lesion: minimal luminal diameter, reference vessel diameter and percent diameter stenosis. Late loss was calculated from the difference between minimal luminal diameter immediately post shielding and at six-month follow-up. Restenosis was defined as the presence of in-lesion >50% diameter stenosis at follow-up.
Fractional flow reserve assessment
Fractional flow reserve was measured with a sensor-tipped 0.014” angioplasty guidewire (WaveWire/WaveMap; Volcano Therapeutics, Inc., Rancho Cordova, CA, USA; or PressureWire; Radi Medical Systems, Uppsala, Sweden). After crossing the target lesion with the wire, hyperaemia was induced with intravenous infusion of 140 μg/kg/min of adenosine (Adrecar; Sanofi, Munich, Germany) for a total of two minutes. The maximum pressure gradient used to calculate FFR was defined as the ratio of the mean post-stenotic pressure to the mean aortic pressure, measured by the guiding catheter, during maximal hyperaemia. FFR of ≥0.75, was considered functionally not significant and constituted the enrolment criterion. Exact FFR measurement at baseline and at six-month follow-up was recorded.
IVUS-VH acquisition and analysis
Details regarding the validation of the technique have previously been reported11,12. Briefly, IVUS-VH uses spectral analysis of IVUS radiofrequency data to construct tissue maps that are correlated with a specific spectrum of the radiofrequency signal and assigned colour codes (fibrous [labelled green], fibrolipidic [labelled greenish-yellow], necrotic core [labelled red] and calcium [labelled white]).
IVUS-VH data was acquired using either the In-Vision Gold console (in the same pullback as palpography) or the S5 imaging system, and a 20 MHz Eagle Eye® Gold catheter (all: Volcano Therapeutics, Inc., Rancho Cordova, CA, USA). The IVUS-VH sampling rate during pullback is gated to peak R-wave and is therefore dependent on heart rate.
IVUS B-mode images were reconstructed from the radio frequency (RF) data by customised software (IVUS Lab Version 4.4; Volcano Therapeutics INC., Rancho Cordova, CA, USA). Semi-automated contour detection of both lumen and the media-adventitia interface was performed and the RF data was normalised using a technique known as “blind deconvolution”, an iterative algorithm that deconvolves the catheter transfer function from the backscatter, thus accounting for catheter-to-catheter variability. Compositional data obtained for every slice was expressed as mean percent for each component.
Pullback of 40 mm was performed after administration of 100-200 micrograms of intracoronary nitroglycerine and incorporated the segment at least 5 mm proximal and distal to the region of interest. Pullback speed was 0.5 mm/sec.
Online analysis was performed to look for IVUS-defined thin-cap fibroatheroma (ID-TCFA) (enrolment criterion). The analysis was subsequently repeated off-line by two independent observers blinded to patient clinical data and randomisation to verify the presence of ID-TCFA. After tracing the lumen and external elastic membrane diameters, plaque, lumen and total vessel area and volumes were computed for the segment of interest. The three consecutive cross-sections with >40% plaque burden and >10% necrotic core in contact with the lumen were identified and their quantitative characteristics and measurements were recorded. In addition, minimal luminal area (MLA) was measured.
IVUS-palpography acquisition and analysis
Intravascular ultrasound palpography is a technique that allows the assessment of local mechanical tissue properties. At a defined pressure difference, soft tissue (e.g., lipid-rich) components will deform more than hard tissue components (e.g., fibrous-calcified)13-15. In coronary arteries, the tissue of interest is the vessel wall, while the blood pressure with its physiologic changes during the heart cycle is used as the excitation force. Radiofrequency data obtained at different pressure levels are compared to determine the local tissue deformation.
Each palpogram represents the strain information for a certain cross-section over the full cardiac cycle. Palpograms will be acquired using a 20 MHz phased-array IVUS catheter (Eagle-Eye®; Volcano Therapeutics Inc. , Rancho Cordova, CA, USA). Cine runs, before and during contrast injection were performed to define the position of the IVUS catheter. Digital radiofrequency data was acquired using a custom-designed workstation.
During the recordings, data was continuously acquired at a pullback speed of 0.5 mm/sec using an automated pullback device (Track Back II; Volcano Therapeutics Inc., Rancho Cordova, CA, USA) with simultaneous recording of the ECG and the aortic pressure. The data was stored on a DVD and sent to the imaging core lab for offline analysis (Cardialysis BV, Rotterdam, The Netherlands).
The local strain was then calculated from the gated radiofrequency traces using cross-correlation analysis and displayed colour-coding, from blue (for 0% strain) via red through to yellow (for 2% strain). This colour-coded information was superimposed on the lumen vessel boundary of the cross-sectional IVUS image.
Using previously described methodology, plaque strain values were assigned a Rotterdam Classification (ROC) score ranging from one to four (ROC I: 0-0.5%; ROC II: 0.6-<0.9%; ROC III: 0.9-1.2%; ROC IV: >1.2%). A cross-sectional area (CSA) was defined as a high strain when it had a high strain region (ROC III-IV) that spanned an arc of at least 12° at the surface of a plaque (identified on the IVUS recording) adjacent to low-strain regions (<0.5%). The highest value of strain in the cross-section is taken as the strain level of the CSA.
Highest strain value pre- and post-shielding was recorded and colocalisation with the IVUS-VH derived TCFA performed using timestamps.
TD and OFDI-OCT acquisition and analysis
The OCT M3 time domain optical coherence tomography (TD-OCT) and C7 optical frequency domain imaging optical coherence tomography (OFDI-OCT) systems used in this study (LightLab Imaging Inc., Westford, MA, USA) have been described previously16-21. Briefly, the OCT catheter was advanced distal to the stented lesion over a conventional coronary guidewire in the case of the C7 system or, in the case of the M3 system, the OCT imaging wire (ImageWire™; Lightlab Imaging Inc., Westford, MA, USA) was directly advanced past the lesion. The OCT catheter was then withdrawn proximal to the stented segment and the lesion visualised using an automated pullback system at 20 mm/sec in the case of the C7 system and 3.0 mm/sec in the case of the M3 system. During image acquisition, coronary blood flow was replaced by continuous flushing of contrast at 3.0-4.0 ml/sec using a power injector (Mark V ProVis; Medrad, Inc., Indianola, PA, USA) at 300 psi. Cross-sectional images were acquired at 100 frames/sec for the C7 and 20 frames/sec for the M3. During the baseline study documentary OCT was performed to measure and record the thickness of the fibrous cap overlying the lipid pool corresponding to the area of the ID-TCFA. A significant lipid pool was defined as a heterogeneous area of attenuated OCT signal, present in more than one quadrant of the vessel wall. The thinnest cap measurement was recorded. The assessment of the shield with OCT post implantation was used to assess procedure-related trauma to the vessel wall (plaque prolapse, presence of filling defects, proximal and distal edge dissection), and at six-months follow-up to assess shield strut apposition and tissue coverage and to measure the thickness of neo-cap. The thickness of the cap was measured every 1 mm within the shielded segment (15 frames per shield) using 360 degree analysis off-line software. In addition, shield areas were measured immediately post-shielding and at six-months follow-up to assess the degree of continued shield expansion with OCT.
A detailed per strut analysis was provided to illustrate the potential advantage of this device in treatment of these necrotic core rich non-obstructive lesions as compared to drug-eluting balloon expandable stents
Measurements were repeated off-line by two independent observers using Lightlabs imaging software.
Follow-up and study endpoints
The primary endpoint of the study was the acute change in the lesion strain pattern immediately after shielding and acute device and angiographic success. Secondary endpoints of the study included: 1) change in the fibrous cap thickness from baseline to six-months post-shielding, 2) change in the stent area, 3) percent diameter stenosis at baseline and at follow-up, late loss and binary restenosis rate, and 4) cumulative incidence of major adverse cardiac events (death, MI and revascularisation) at six-month follow-up. Stent thrombosis occurrence was defined and classified according to the Academic Research Consortium (ARC) criteria22.
Sample size calculation and statistical analysis
The study population was statistically based on the change in study lesion strain patterns immediately post-stenting, as noted in the ABSORB trial23. In this trial the mean of the maximal strain/cross-section/patient decreased from 0.44±0.25 to 0.00±0.01. Based on the assumptions for these, the sample size was calculated as detailed below.
Assumptions for the sample size calculation using a paired t-test:
– mean difference between pre- and post-treatment equal to zero
– alpha=0.05;
– mean pre=0.4;
– mean post=0.0;
– SD of difference pre-post=0.3;
– 90% power.
To assess the change in strain observed on palpography post-treatment, paired (pre-and post-) data of nine patients would have been needed. However, in order to account for the patients lost to follow-up, we aimed to enrol a total of 15 patients in each arm of the trial.
Discreet variables are presented as counts and percentages. Continuous variables are expressed as means±standard deviations.
Results
Patient enrolment
From June 2008 until February 2010 over 100 patients were approached for participation in the trial. Forty-eight signed informed consent, but only 23 patients met inclusion and enrolment criteria (including presence of ID-TCFA) and were enrolled in the trial. Thirteen patients were randomised to shield device and 10 randomised to medical therapy but with one patient crossing over to the shield arm. Baseline clinical characteristics of the patients enrolled are summarised in Table 1. Notably 24% of the patients were diabetic and 65% had multivessel disease. Of the 13 shielded patients, 11 completed full angiographic and imaging follow-up. Of the 10 control patients only five completed full angiographic and imaging follow-up.
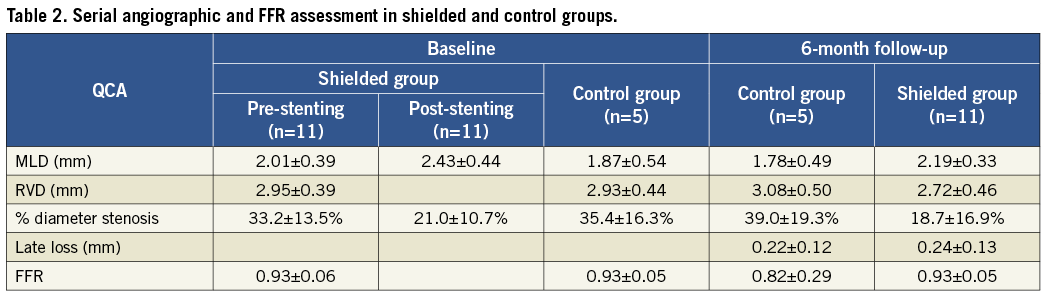
Angiographic and FFR analysis
In 24% of the cases, proximal or mid left anterior descending (LAD) artery was the site of the TCFA , in 24% the left circumflex LCx coronary artery and in 52% cases the right coronary artery (RCA). In the shielded group, baseline percent diameter stenosis was 33.2±13.5% with minimum lumen diameter (MLD) of 2.01±0.39 mm (Table 2). Baseline FFR was 0.93±0.06. Post-stenting percent diameter stenosis decreased to 21.0±10.7 in the shielded patients and MLD increased to 2.43±0.44 mm. At six-month follow-up in shielded patients, percent diameter stenosis further decreased to 18.7±16.9% with MLD of 2.19±0.33 mm and FFR remained the same (0.93±0.05). Average late loss was 0.24±0.13 mm. FFR in the control group at six months was 0.82±0.29 compared to 0.93±0.05 at baseline.
IVUS-VH analysis and palpography
At the site of the TCFA lesion baseline plaque burden was 60.6±8.8%, percent necrotic core in contact with the lumen was 34.7±6.3% averaged over three consecutive frames. Average MLA was 6.8±2.4 mm2 (Table 3 and Figure 3). At follow-up, the five control patients showed no increase in plaque burden or necrotic core observed over time and no MLA decrease.
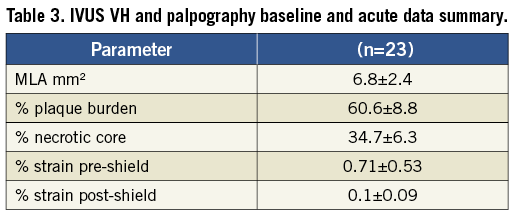
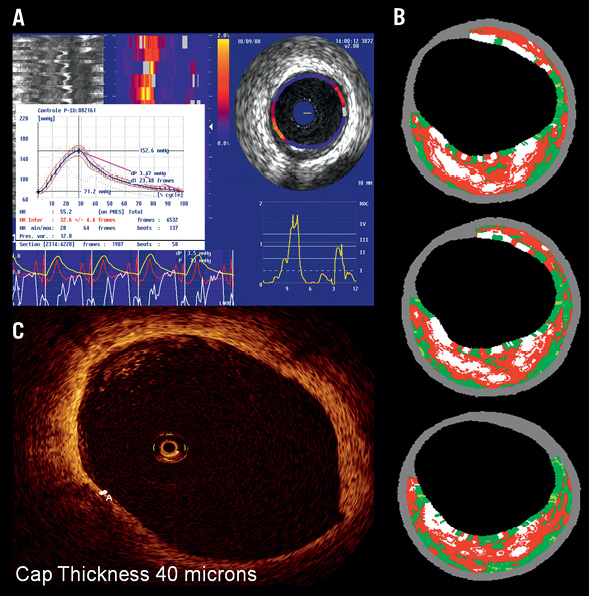
Figure 3. Example of baseline imaging for one of the enrolled patients. A) In the upper left, palpogram showing stain value of 1.4% (ROC III-IV); B) In the upper right corresponding matched TCFA on IVUS VH analysis with plaque burden of 56% and necrotic core of 34% in three consecutive frames; C) In lower left corner, matched OCT frame showing cap thickness of 40 µm.
Average strain before shield placement was 0.71%±0.53% (ROC score of II on average). This decreased acutely post-shield placement to 0.1%±0.09% (ROC score of I).
OCT analysis and data
As previously reported by our group24, deployment of the self-expanding shield resulted in minimal trauma to the vessel wall, particularly when compared to the balloon-expandable devices. There were no proximal or distal edge dissections and no filling defects. Length of intra-stent dissections was also minimal.
Average baseline fibrous cap thickness was 48±12 µm with a range of 30-70 µm. After shield placement at six-month follow-up neo-cap formation was observed with average cap thickness of 201±168 µm (range 50-608 µm) (Table 4). The patient with 608 µm of neo-cap formation at baseline had adjacent calcifications that required high pressure (16 atms) post-dilation of the shield with resultant barotrauma and more exuberant healing response.
In addition, mean stent area of 8.76±2.16 mm2 increased to 9.45±2.30 mm2, that is by 9% at six-month follow-up (Table 4 and Figure 4). The number of malapposed struts decreased from 10.7% to 7.6% and the number of uncovered struts at six months was 8.1%.
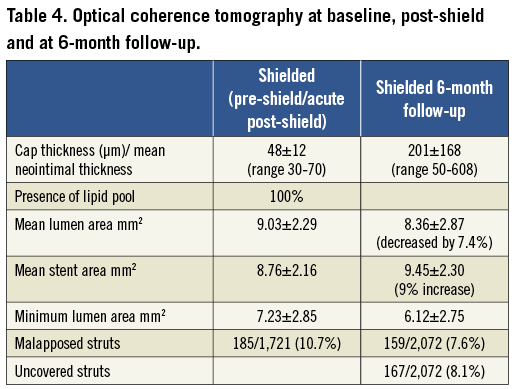
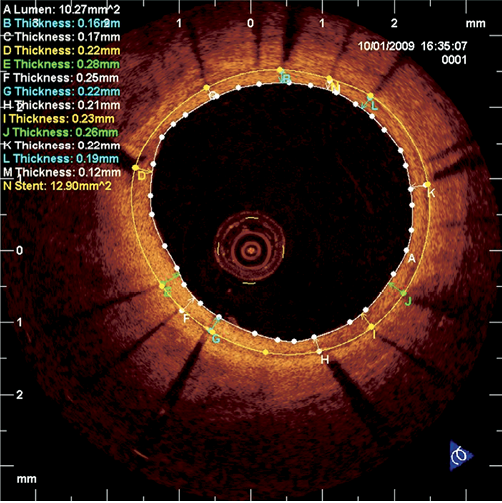
Figure 4. Example of per-strut OCT analysis and appearance of vShield at six-month follow-up with uniform strut coverage of around 200 µm and no malapposition.
Detailed per strut analysis
A total of 11 stents were evaluated at baseline. In two patients there was a high degree of malaposition due to undersizing of the device. Mean incomplete stent apposition (ISA) area was 0.36±0.47 mm2. Mean prolapse area was 0.009±0.17 mm2. Of the 1,721 stent struts counted at baseline 1,521 were well apposed, 185 (10.7%) were malapposed and 15 were in front of side branches. There were no dissections seen. Mean thrombus area was 0.015 mm2.
At six-month follow-up 12 stents were evaluated with a total length of 142.95 mm. Mean lumen area was 8.36±2.87 mm2 (decreased by 7.4%). Mean stent area increased to 9.45±2.30 mm2 (by 9%), implying continued stent expansion. Mean ISA area was 0.88±0.85 mm2. Of the total of 2,072 struts evaluated, 1,910 were well apposed, 159 were malapposed (7.6%; decrease from baseline), and three were in front of a side branch. Of all struts 8.1% were non-covered. Of the well-apposed struts, 93.2% were covered, while of the malapposed struts 78% were covered.
Clinical events
There were no device-related MACE events (Table 5). One of the control (non-shielded) patients returned within two weeks of the procedure with an unstable coronary syndrome and crossed over to the shield arm. There were no stent thrombosis events. Lastly, non-invasive assessment of shield patency with MSCT appears feasible owing to its thin nitinol struts (Figure 5).
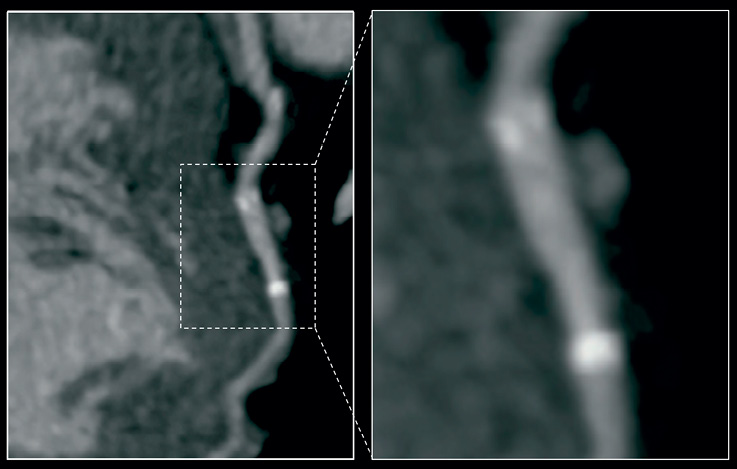
Figure 5. MSCT image of vShield at six months. There is no beam-hardening artefact from nitinol struts (except for tantalum markers at the edges) allowing for good non-invasive evaluation of patency.
Discussion
In this first-in-man experience with shielding of vulnerable plaque (thin-cap fibroatheroma) using a self-expanding nitinol shield, we demonstrate the feasibility and preliminary efficacy of the approach. The device delivery was successful in all 13 patients who were randomised to the shield and there were no MACE events related to the shield device treatment at six-month follow-up. The treatment strategy employed in this protocol is based on the fact that most myocardial infarctions (MI) result not from a critical blockage, but from lesions that are non-flow limiting25-30. In individuals who have undergone angiography in the months preceding myocardial infarction, the culprit lesions most often show <50% diameter stenosis27. Moreover, it has been shown on a previous angiogram that only approximately 15% of acute MI arise from lesions of <60% stenosis11. These lesions, however, have a substantial plaque volume/percent plaque burden. The coronary flow is not obstructed because of outward (positive) remodelling. Longer-term prognosis of a patient might depend on far more detailed plaque assessment than angiography and on adequate treatment of plaques at risk of rupture.
The use of IVUS-VH to identify vulnerable plaques (ID-TFCA) is well documented and is comparative to what has been demonstrated from documented plaque ruptures. ID-TCFA is currently defined as a lesion fulfilling the following criteria in at least three consecutive cross-sectional areas (CSA): 1) necrotic core ≥10% without evident overlying fibrous tissue, 2) lumen obstruction ≥40%. In addition, the ID-TCFA must demonstrate positive remodelling by having a remodelling index (RI) >1.05. In a study population of 21 patients Garcia-Garcia12 found, in 13 patients, 42 ID-TCFA that fulfil the IVUS-VH criteria. This meant that on average there are approximately three ID-TCFA per patient. Documented plaque ruptures were reported by Rioufol31 in 2002 in 24 patients referred for PCI after a first acute coronary syndrome (ACS) with a troponin I elevation. He found that there were 50 plaque ruptures corresponding to 2.08 vulnerable plaques per patients presenting with an ACS, which is in accordance with Garcia-Garcia’s IVUS-VH findings. Interestingly, plaque rupture on the culprit lesion was found only in nine patients (37%). In 19 patients (79%) at least one plaque rupture was found somewhere other than the culprit lesion, in a different artery in 70% and in both other arteries in 12.5% of the patients. This reinforces the importance of identifying and treating vulnerable plaques and the fact that they can be remotely associated from the culprit lesion causing the presenting symptom. This also constitutes the rationale for the treatment of intermediate non-flow limiting lesions with signs of vulnerability. Accuracy of thin-cap atheroma detection can be further increased by combining IVUS-VH imaging with OCT imaging of the lesion, which due to its micron resolution can allow the measurement of the thickness of the fibrous cap. Sawada and Garcia-Garcia32 have shown that out of 126 lesions examined with two modalities only 28 (22%) fulfil thin-cap fibroatheroma criteria by both IVUS-VH and OCT with thin cap defined as <65 microns. For these reasons, we have chosen in this study to perform a very detailed multimodality examination of plaque before enrolling patients in the study. The examinations that each patient underwent were: 1) angiography, 2) FFR, 3) palpography (off-line), 4) IVUS-VH, and 5) OCT online at baseline. This was followed by post-shielding assessment with: 1) angiography, 2) palpography, and 3) OCT. At six-month follow-up the assessment included: 1) angiography, 2) FFR, 3) palpography/IVUS, and 4) OCT. With such extensive examination and procedure times, which was challenging for patients, personnel and operators, enrolment in the study was rather slow (23 patients in under two years), and several patients (particularly in the control arm) were unwilling to participate in the follow-up catheterisation. The use of stringent criteria for enrolment was justified in this pilot study; the protocol may have been more successful had we used a simple combination of non-invasive coronary MSCT assessment (for positive remodelling, plaque burden, 3-D strain and flow) combined with intraprocedural OCT (to measure cap thickness and show presence of a lipid pool). In the future, angiography, FFR and IVUS/palpography assessment should be replaced by non-invasive methodologies such as MSCT or combined MSCT-FDG-PET examination33 which after evaluation against invasive technologies could potentially provide equivalent information before the start of the invasive procedure34-36.
We have been able to demonstrate here that the self-expanding device is ideally suited for treatment of thin-cap fibroatheromas. The self-expanding nature of the device causes minimal trauma to the vessel wall, minimising the risk of thin-cap rupture and necrotic core embolisation. We had no periprocedural MI in this patient cohort. Furthermore, the device is well apposed and continues to expand gently by 9% over six months, minimising the risk of having malapposed and uncovered struts. While there is no drug coating and the device is bare metal, the combination of thin nitinol struts and lack of traumatic balloon expansion result in minimal neointimal formation. Eight percent of the struts were still uncovered at six months with average neo-cap of 201 µm and late loss of 0.13 mm which is comparable to some of the state-of-the-art drug-eluting stents. There were no stent thrombosis events. The continued gentle expansion of the device is similar to that observed by Granada et al in the first-in-man trial of the vShield device in moderate stable lesions, which was completed recently37 and also comparable to the results achieved with the Stentys stent (STENTYS Inc., Princeton, NJ, USA) in the Apposition study38.
The number of patients enrolled and lack of events made it impossible to determine whether placement of the shield and plaque passivation demonstrated by OCT offered an advantage over standard medical therapy with aspirin, clopidogrel and statins. The ability to prevent plaque growth and disease progression to a significant lesion was demonstrated recently in the VELETI trial of paclitaxel-eluting stent treatment versus medical therapy in graft disease10.
Limitations
The present report is a pilot study and the number of patients is limited, and should therefore be considered exploratory and hypothesis-generating, without formal statistical hypothesis.
The limited number of patients made any meaningful statistical analysis rather difficult and thus the data are presented for most part in a qualitative fashion.
Moreover, an important limitation was failure to complete the full projected study enrolment and lack of angiographic/imaging follow-up in a large proportion of non-shielded control arm patients. In addition, since only 4.4% of the VH-derived TCFA lesions result in event rates at three years based on the finding of the PROSPECT study9 (in the absence of MLA<4 mm2 or >70% plaque burden), despite our extensive use of imaging such as concomitant OCT we may have failed to identify truly high-risk plaques.
Conclusion
Passivation of the thin-cap fibroatheroma with a self-expanding nitinol vShield device appears to be safe and feasible. A larger cohort study with long-term follow-up will be needed to evaluate this device as a treatment for necrotic core rich lesions.
Conflict of interest statement
In the past, J. de Schepper was an employee of Prescient Medical. The other authors have no conflicts of interest to declare.

