Abstract
Aims: To determine whether the efficacy of FX06 was dependent upon the timing of reperfusion therapy or the presence of collaterals in the Efficacy of FX06 in the prevention of myocardial reperfusion injury (F.I.R.E.) trial.
Methods and results: Two hundred and thirty-four (234) patients presenting with acute ST-segment elevation myocardial infarction were randomised to FX06 or matching placebo given as an intravenous bolus at reperfusion. Infarct size was assessed at 5-7 days and four months after myocardial infarction by cardiac magnetic resonance imaging determined total late enhancement and necrotic core zone. Patients were stratified according to presentation status (time-to-therapy <3 hours, n=108; time-to-therapy=3-6 hours, n=115) and presence of collaterals (yes, 46; no, 177). There were no statistically significant differences between groups at day 5-7. At four months, we observed statistically significant reductions of both measures of infarct size (0.3% vs. 2.4%, p=0.038; 8.0% vs. 16.0%, p=0.032) in the group given FX06 and presenting early. There was also a statistically significant reduction of total late enhancement zone among patients given FX06 with collaterals (7.3% vs. 15.2%, p=0.043). No differences were evident among late presenters or those without collaterals.
Conclusions: FX06 significantly reduced infarct size at four months in the early presenters and in those with collaterals.
Abbreviations
PCI: Percutaneous coronary intervention
MI: Myocardial infarction
STEMI: ST elevation myocardial infarction
TIMI: Thrombolysis in myocardial infarction
CMR: Cardiac magnetic resonance
cTnI: cardiac troponin I
LV: Left ventricle
SD: Standard deviation
ECG: Electrocardiogram
LVEF: Left ventricular ejection fraction
Introduction
The recently concluded F.I.R.E. (Efficacy of FX06 in the prevention of myocardial reperfusion injury) trial investigated whether infusion of a naturally occurring peptide derived from human fibrin (sequence Bß15-42), FX06, as an adjunct to primary PCI would reduce myocardial infarct size by mitigating the reperfusion injury1. Lethal reperfusion injury, first described in 1960 by Jennings, limits the benefits of reperfusion therapy by inducing necrosis of cardiomyocytes injured by the ischaemic insult, but still viable at the time of re-establishment of the perfusion2-4. In some animal models reperfusion injury accounts for up to 50% of final infarct size4. Leukocyte infiltration has long been thought to play one of the key roles in conveying reperfusion injury5-7. FX06, by binding to vascular endothelial cadherin, is thought to inhibit the last and irreversible step in leukocyte diapedesis, the actual transmigration through endothelial junctions and into the injured tissue8.
The primary endpoint of F.I.R.E., infarct size at five days as determined by CMR, demonstrated no statistically significant difference between the placebo and FX06 treated patients. However, the necrotic core zone of the affected myocardium was reduced at five days by a statistically significant margin. Four months follow-up revealed no significant differences between the two groups in terms of infarct size, though the results all tended in favour of the active-drug arm. Importantly, the drug also appeared well tolerated and safe.
Time-to-therapy, as an expression of the severity of ischaemia, has been proposed to be an important determinant of the efficacy of interventions aimed at mitigating reperfusion injury2,7. This concept is both biologically plausible and supported by previous data9. Therefore, in the design of the F.I.R.E. trial it was considered of importance to include a pre-specified substudy to define the efficacy of FX06 in patients stratified by presenter status. Hypothesising that the degree of ischaemia is a determinant of the efficacy of FX06, one would also expect that those patients with collateral blood supply to the area affected by ischaemia would benefit disproportionately from the drug. Consequently, the present sub-analysis aimed at investigating the possible differential efficacy of FX06 as a function of time-to-reperfusion and presence of collaterals.
Methods
Trial
The F.I.R.E. trial was an international, multicentre, randomised, double-blind, placebo-controlled study (NCT00326976 at clinicaltrials.gov). Details of the rationale and design have been described elsewhere1,10.
Patients
The study population consisted of patients presenting with a first STEMI and no other serious comorbidities. Additional inclusion criteria were presentation within six hours from onset of symptoms, >2 mm ST-segment elevation in at least three ECG leads, and a single culprit lesion with TIMI flow 0/1 in the infarct-related artery. Patients with prolonged ischaemic symptoms, cardiogenic shock, peripheral vascular disease and history of kidney or liver dysfunction were excluded. All patients were treated for STEMI per current guidelines. FX06 (400 mg) was administered in two intravenous bolus injections of 200 mg each during PCI, the first immediately before the guide wire passed the occlusion and the second 10 min (±5 min) later. Concomitant therapies were allowed except for thrombolytics and adenosine. All patients were followed clinically for four months and CMR images were obtained at5-7 days and four months past the index event. Prespecified subgroups included early and late presenters. Early presenters refer to those patients with a maximum of <3 hours since onset of symptoms until re-establishment of blood flow (time-to-therapy), while late presenters refer to those patients with time-to-therapy between three and six hours. Collateral blood supply was determined angiographically by the Rentrop scale (≥1). Written informed consent was obtained from all patients. The ethics review board for each respective participating centre approved the protocol, and the study was done in accordance with the Declaration of Helsinki.
Biomarker measurements
Cardiac troponin I (cTnI) were sampled at 24 and 48 hours. All cTnI samples were analysed in a blinded core laboratory (Spranger Laboratories, Ingolstadt, Germany). Cardiac troponin I (cTnI) was measured on the Abbott AxSym® System (Abbott Diagnostics, Abbott Laboratories. Abbott Park, IL, USA) using the second-generation AxSym® Troponin-I ADV assay.
Imaging
Infarct size was determined by contrast enhanced CMR imaging by infusion of gadolinium contrast. The evaluation of infarct size was done blinded in an independent core laboratory (Professor P. Buser, University Hospital, Basel, Switzerland). The details of the imaging procedure and the subsequent assessment of the images have been described in detail in the original paper1. In brief, the different areas of the infarct zones were defined in the following manner: Areas with very bright signal, i.e., white areas, were designated as the necrotic core zone, where the majority of myocytes were irreversibly injured. In the setting of acute MI, this necrotic bright area often contains a dark core, designated as microvascular obstruction zone where contrast medium penetration is precluded. Areas of microvascular obstruction were included in the measurement of the necrotic core zone. In addition to these two tissue zones (necrotic core and microvascular obstruction zone), an infarct zone was detected with signal intensity higher than in the normal myocardium with a “nulled” signal, but lower than in the necrotic core zone. All three tissue compartments were separately measured, and the total volumes were obtained by summing the compartments of all slices covering the entire LV. In the present paper, total late enhancement will refer to the three compartments considered together. For assessment of the impact of FX06 on infarct size, both the necrotic core and total late enhancement measurements were considered. Infarct size is expressed relative to total LV mass.
Statistics
Sample size calculations in the main trial for the primary endpoint were based on an expected infarct size of 18% of the left ventricle in the placebo group. With an expected reduction of 25% and a common SD of 11%, 95 patients in each group were required to detect a 4.5% treatment difference with a 2.5% 1-sided significance and 80% power. All efficacy results were reported for the intent-to-treat population. All adverse events were reported for the safety population. Due to their highly skewed distribution, myocardial infarct size data and cTnI measurements were summarised as median (interquartile range) and compared using the non-parametric two-sided Wilcoxon’s rank sum test. LVEF measurements approximated a normal distribution and were consequently presented as mean (standard deviation) and compared by Student’s t test. Statistical significance was assumed with two-sided p-values of <0,05. All statistical analyses were performed by an independent and external entity (IFE, Essen, Germany) using SAS (SAS Institute Inc, Cary, NC, USA).
Results
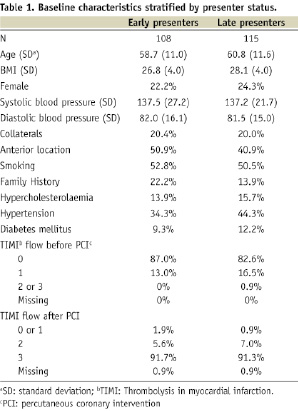
Two hundred and thirty-four (234) patients were randomised. Seven were determined not to be eligible (three, not first MI; three, no evidence of MI on baseline ECG; one, cardiogenic shock). Consequently, the intent-to-treat population consisted of a total of 227 patients and of these, four patients were excluded because information on time-to-therapy was missing. One hundred and eight (108) patients (FX06, 53; placebo, 55) were treated within three hours and subsequently included in the early presenter group. One hundred and fifteen (115) patients (FX06, 55; placebo, 60) received PCI between three and six hours and were stratified to the late presenter group. Forty-six (46) patients were determined to have collateral blood supply, while 177 did not. Baseline features of the early and late presenters are listed in Table 1.
Baseline features of the placebo versus FX06 treated patients in the early presenter and late presenter groups are listed in Table 2.
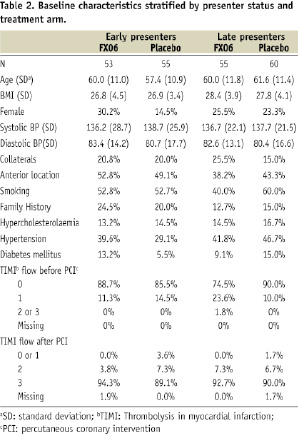
In general the pre-specified subgroups were similar in terms of baseline characteristics. However, there were substantial differences with regard to family history of coronary artery disease, hypertension and infarct location. About half of the patients presented with an anterior infarct and overall, approximately 1/5 of the patients had collateral blood supply to the infarcted area. There were no differences in mean CMR measures of infarct size between the groups. However, the distribution was highly skewed with a few large outliers. Therefore, data are presented as medians and nonparametric statistical tests were employed for infarct size measures.
Infarct size
There were no statistically significant differences in CMR measures of infarct size among early or late presenters at 5-7 days past the index-event (Figure 1 and 2). At four months follow-up, both measures of infarct size (necrotic core zone and total late enhancement) were significantly reduced among FX06 treated patients compared to control patients in the early presenters. In patients presenting late, there were no statistically significant differences in measures of infarct size.
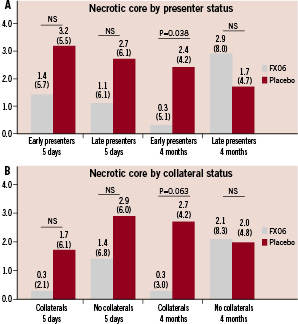
Figure 1. Necrotic core zone (as % of left ventricle) at 5-7 days and four months for patients stratified by presenter status (< 3 hours vs. 3-6 hours) (A) and presence of collaterals (B). Results presented as medians (interquartile ranges).
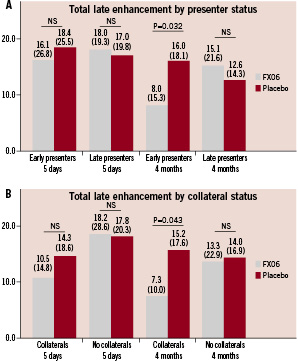
Figure 2. Total late enhancement zone (as % of left ventricle) at 5-7 days and four months for patients stratified by presenter status (<3 hours vs. 3-6 hours) (A) and presence of collaterals (B). Results presented as medians (interquartile ranges).
Among patients with collateral blood supply to the infarct area, no statistically significant difference in infarct size at 5-7 days were present. At four months follow-up, infarct size expressed as total late enhancement was significantly reduced among the FX06 treated patients, while the necrotic core zone reductions tended heavily in favour of FX06 but did not reach statistical significance. In patients without collaterals, no statistically significant changes were evident either at 5-7 days or four months.
Left ventricular ejection fraction
Marginal and non-significant differences were evident in LVEF at 5-7 days and four months when patients were stratified according to both presenter and collateral status (Figure 3).
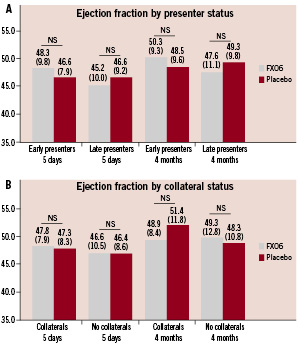
Figure 3. Left-ventricular ejection fraction (%) at 5-7 days and 4 months for patients stratified by presenter status (<3 hours vs. 3-6 hours) (A) and presence of collaterals (B). Results presented as means (standard deviations).
Cardiac troponin I
There were non-significant reductions of cTnI values among both early and late presenters given FX06 when compared to the control group (Figure 4). In patients treated with FX06 with collateral blood supply we observed substantial reductions of cTnI release which were borderline significant at 24 hours and achieved significance at 48 hours. No differences were evident among patients without collateral blood supply.
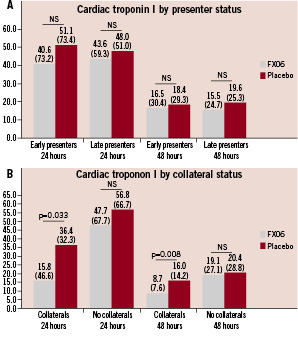
Figure 4. Cardiac troponin I concentrations (ng/ml) at 24 and 48 hours for patients stratified by presenter status (<3 hours vs. 3-6 hours) (A) and presence of collaterals (B). Results presented as medians (interquartile ranges).
Clinical endpoints
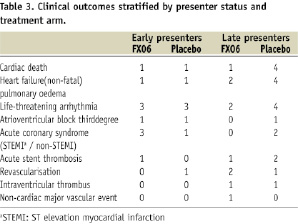
No differences were seen in cardiovascular-related serious adverse events when patients were stratified according to presenter status (Table 3). However, there was a distinct trend in favour of FX06 compared to placebo with regard to new onset heart failure and cardiac death among the late presenters.
Discussion
The present prespecified subanalysis of the F.I.R.E. trial aimed at investigating the potential cardioprotective properties of FX06 as a function of time-to-therapy and presence of collaterals in the setting of an acute myocardial infarction. The results suggest a signal of an augmented efficacy of FX06 in those presenting early (time-to-therapy <3 hours) and/or those with collaterals present. Despite the relatively small number of patients in each cohort, the differences between FX06 and placebo in the above mentioned groups attained statistical significance on both measures of relative infarct size at four months. The results should be interpreted with caution and only considered hypothesis-generating.
Even though the concept of reperfusion injury is well-established in experimental studies6 cardio protection from reperfusion injury has proved an elusive target in clinical trials9,11-14. The reasons for the lack of correspondence between animal and human trials are likely manifold and complex. Several reports have suggested that the duration and burden of ischaemia might play a key role15,16. Specifically, a number of previous animal trials have signalled that an anti-neutrophil strategy for combating reperfusion injury is extremely reliant on an early intervention to be successful7,17,18. Also in agreement with these observations is the indication from former studies that anti-neutrophil strategies are more likely to succeed in dogs, known to have substantial collateral blood flow which would, in effect, prolong the period of salvageability17,19,20. FX06 inhibits leukocyte diapedesis and has also recently been shown to strengthen the endothelial barrier function8,21. It is conceivable that the mechanisms by which FX06 conveys cardio protection are rendered ineffective after prolonged coronary occlusion where the ischaemia would compromise the integrity of the endothelial barrier. Thus, the a priori formulation of the hypothesis tested in the present investigation was founded on empirical evidence and theoretical considerations; and our results should be interpreted in light of these above concepts.
Although a clear trend favouring FX06 treated subjects with regard to infarct size was evident at five days, the statistically significant differences were only present at four months. Oedema, haemorrhage and cellular debris affect the delineation of infarct size at five days post the index event22. It is possible that these phenomena have blunted the differences between placebo and FX06 groups at 5-7 days. Furthermore, although FX06 was only administered at reperfusion, it affects processes that are likely to impact infarct size evolution for a much longer time-span7. Such non-linear dynamics are ubiquitous in biology23. Nevertheless, these interpretations remain speculative. Also, placebo groups with collaterals or presenting early experienced little or no infarct size involution22. This would seem to increase the possibility that our findings to some extent are due to chance. Additionally, 29 patients did not return for a follow-up CMR examination which also might have distorted the findings at four months. We did not observe statistically significant differences between the placebo and active drug arm in left-ventricular ejection fractions. Given the limited size of the F.I.R.E. population, and considering that these were first-time myocardial infarction patients, this was expected and is furthermore in keeping with results of other recent trials also failing to demonstrate significant improvement of functional parameters, despite reductions of infarct size24,25.
Despite the large number of disappointing clinical trials, achievement of cardio protection from reperfusion injury is still considered a promising avenue of further research with the aim of advancing outcomes in patients with STEMI. Our report provides more insight into the results of the F.I.R.E. trial, although no direct clinical implications are suggested by this exploratory substudy. Yet, by offering a further indication of the narrow therapeutic window that exists for amelioration of reperfusion injury, the results yield hypotheses that could facilitate further research and comprehension of reperfusion injury.
This study has several and important limitations. Subpopulations were small, which resulted in some differences between groups at baseline and limits the statistical power of the analyses. Furthermore, all the efficacy endpoints were surrogate markers. However, infarct size has been demonstrated to be an important determinant of subsequent clinical events following STEMI26. There was a loss to follow-up at four months of about 15% of patients which might have produced a biased analysis. CMR images were obtained at 23 different centres and although each participating centre was pre-evaluated, the image quality varied which might have impacted the interpretation of the results. Furthermore, no information was available for treatment during the follow-up period.
Conclusion
Our results indicate that FX06 given in conjunction with primary PCI within three hours after onset of symptoms in the setting of STEMI results in substantial, statistically significant and lasting reductions of infarct size. However, the results should only be considered hypothesis generating and larger-scale studies are needed to corroborate our findings.
Acknowledgements
The authors would like to thank Jens Bremerich (University Hospital Basel) for reading the cardiac magnetic resonance images. We thank the physician investigators who contributed to patient enrolment, together with the nursing and technical staff at each participating centre. We would also like to thank Marcos Marin-Galiano for statistical assistance.

