
Abstract
Aims: We aimed to assess the effect of exenatide treatment as an adjunct to primary percutaneous coronary intervention (PCI) on long-term clinical outcome.
Methods and results: We performed a post hoc analysis in 334 patients with a first STEMI included in a previous study randomised to exenatide (n=175) or placebo (n=159) as an adjunct to primary PCI. The primary endpoint was a composite of all-cause mortality and admission for heart failure during a median follow-up of 5.2 years (interquartile range: 5.0-5.5). Secondary endpoints were all-cause mortality and admission for heart failure, individually. The primary composite endpoint occurred in 24% in the exenatide group versus 27% in the placebo group, p=0.44 (HR 0.80, p=0.35). Admission for heart failure was lower in the exenatide (11%) compared to the placebo group (20%) (HR 0.53, p=0.042). All-cause mortality occurred in 14% in the exenatide group versus 9% in the placebo group (HR 1.45, p=0.20).
Conclusions: In this post hoc analysis of patients with a STEMI, treatment with exenatide at the time of primary PCI did not reduce the primary composite endpoint or the secondary endpoint of all-cause mortality. However, exenatide treatment reduced the incidence of admission for heart failure.
Abbreviations
GLP-1: glucagon-like peptide-1
HR: hazard ratio
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
Reperfusion by primary percutaneous coronary intervention (PCI) improves outcome in patients with ST-segment elevation myocardial infarction (STEMI)1,2. However, the five-year cardiac mortality is still 12% and the incidence of heart failure over the same period is 10% for these patients3,4. Hence, further improvement in the treatment is warranted. Despite timely and successful primary PCI treatment, reperfusion itself may damage the myocardium, a phenomenon known as reperfusion injury5,6. During the last decades numerous cardioprotective strategies have focused on preventing or reducing reperfusion injury6-11. One approach is treatment with glucagon-like peptide-1 (GLP-1), which is an incretin hormone that regulates plasma glucose, or its artificial analogues12-14. In an original proof-of-concept study, we found that treatment with the GLP-1 analogue exenatide administered prior to reperfusion by primary PCI increases the myocardial salvage index by 15% in an enriched STEMI population of patients with Thrombolysis In Myocardial Infarction (TIMI) flow 0/1 and single-vessel disease7. Exenatide treatment further decreased final infarct size by 30% in STEMI patients with TIMI flow 0/1 and system delay <132 minutes15. This cardioprotective effect of exenatide on surrogate markers has been confirmed in two other randomised clinical studies16,17. Based on these previous findings, we hypothesised that the cardioprotective effect on myocardial salvage might translate into improved long-term clinical outcome. Therefore, in this post hoc analysis we present long-term clinical outcome subsequent to injury of the left ventricular function in STEMI patients randomised to receive either exenatide or placebo prior to primary PCI7,15.
Methods
STUDY PARTICIPANTS AND TRIAL
This is a post hoc analysis of a randomised, double-blind, placebo-controlled trial evaluating the cardioprotective effect of exenatide treatment performed at Copenhagen University Hospital, Rigshospitalet, Denmark, and Aarhus University Hospital, Skejby, Denmark. The method has been described previously7,15. Briefly, all patients with a suspected first STEMI and symptom duration ≤12 hours were enrolled. STEMI was defined as ST-segment elevation in two contiguous ECG leads of 0.1 mV in V4-V6 or limb leads II, III and aVF, or 0.2 mV in leads V1-V3. Patients with multivessel disease and TIMI 2/3 were excluded in the original study, but to increase the statistical power all patients regardless of the presence of multivessel disease and epicardial flow were included in the present analysis.
All patients eligible for primary PCI were treated according to international guidelines. Medication and primary PCI procedure have been described in the previously published papers7,15. Patients were informed verbally and in writing, and all gave their written consent before inclusion. The study was performed according to the Helsinki Declaration of Good Clinical Practice, and The Danish National Committee on Biomedical Research Ethics approved the protocol. The study was registered at www.clinicaltrials.gov, identifier: NCT00835848. Exenatide was administered intravenously prior to the diagnostic angiography in order to obtain a suitable plasma concentration before reperfusion.
TRIAL PROTOCOL
Randomisation to either placebo or exenatide was performed prior to angiography in all patients without pre-angiographic exclusion criteria using a 1:1 computer-generated sequence. Hence, all patients included in the present study received either exenatide or placebo. The reason why patients excluded from the original study received the full study medication was to assess if any side effects were present. We therefore continued the infusion for the full six hours in all patients, irrespective of angiography. Operator and patient were blinded to the allocated treatment. Patients assigned to exenatide were treated with an intravenous infusion of exenatide BYETTA® (AstraZeneca, London, United Kingdom) of 0.12 μg/min for the first 15 min, and then 0.043 μg/min for 6 hrs in order to maintain an exenatide plasma concentration between 0.03 and 0.3 nmol/L7,14. Patients in the control group were treated with continuous infusion of saline with similar infusion velocities and durations. We did not observe any serious adverse events and there were no hypoglycaemic events.
ENDPOINTS
Outcome data were collected on all patients randomised in the original study with a verified STEMI from Danish nationwide medical registries. This database has collected all hospital data since 1977 and has proven accurate18. All patients were followed from date of inclusion until death or December 2014. At birth, Danish citizens are given a unique identification number. This identification number can be used to link data in registries19. A reviewer blinded to all clinical data evaluated all re-admissions during the follow-up period and evaluated the hospital files for the reason underlying the hospitalisation.
The composite endpoint was a composite of all-cause mortality and admission for heart failure. Secondary endpoints were all-cause mortality or admission for heart failure. All-cause mortality was defined as death from any cause in the follow-up period. Cause of death was obtained from the Danish Causes of Death Registry20. Admission for heart failure was defined as sudden onset of symptoms of heart failure requiring an unplanned visit to the emergency room or hospitalisation and accompanying verified decreased cardiac output (in the absence of other causes such as sepsis), as determined by pulmonary oedema or peripheral enema or insertion of a cardiac resynchronisation therapy (CRT) device or an implantable cardioverter defibrillator (ICD). The need for and insertion of an ICD/CRT was evaluated according to current guidelines and was included in the present definition of admission for heart failure to increase statistical power, since a large proportion of ICD/CRT events are heart failure related21. A medical doctor who did not know study-group assignments examined mortality cause outcomes. Experienced sonographers assessed LV function with echocardiography using the Philips IE33 Ultrasound System (Philips Healthcare, Best, The Netherlands). CMR was performed as previously described using a 1.5 T scanner (Avanto scanner; Siemens, Erlangen, Germany)7.
STATISTICS
Binomial variables are expressed as sum (% of total). All continuous variables are expressed as mean (standard deviation) unless otherwise stated. Categorical variables were compared with the chi-square test or Fisher’s exact test. Continuous variables were evaluated for normal distribution and compared using the Student’s t-test or the Mann-Whitney test. Cox proportional hazards regression analyses were used to calculate hazard ratios (HR) with 95% confidence intervals (CI). The Kaplan-Meier method was used for visual assessment of the cumulative incidence of events and p-values calculated with the log-rank test. A two-tailed p-value <0.05 was considered statistically significant. All statistical analysis was performed using IBM SPSS Statistics, Version 20.0 (IBM Corp., Armonk, NY, USA) and R version 3.0.3 (R Development Core Team 2014, http://www.R-project.org/).
Results
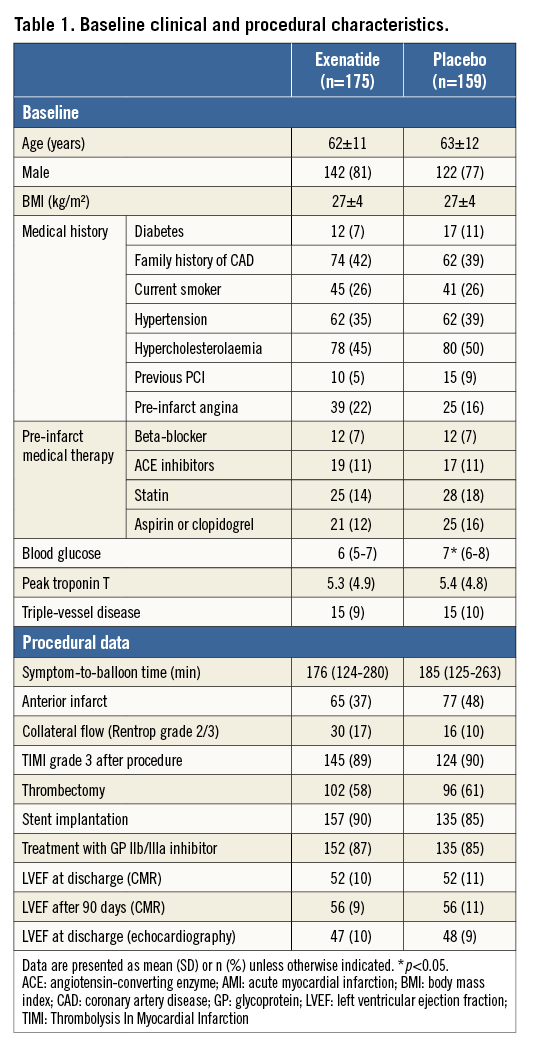
A total of 578 consecutive patients presenting with STEMI and admitted to the catheterisation laboratories were screened for inclusion. One hundred and ninety-one patients did not meet inclusion criteria due to the various reasons listed in Figure 1; 387 patients were randomised in the original study, but a further 52 who had no myocardial infarction (21 patients in the exenatide group and 31 patients in the placebo group) and one patient who withdrew consent were therefore excluded. This left a total of 334 patients to be included in the present study (175 in the exenatide group and 159 in the placebo group, with no significant difference of distribution between groups, p=0.41). Figure 1 shows the study flow chart. Table 1 shows the baseline demographics and procedural results for the randomised patients with AMI. The treatment groups were overall well balanced and no gender-based differences were present. There were no differences in any heart failure parameters (echocardiography or CMR assessed left ventricular ejection fraction). As expected, exenatide treatment resulted in lower levels of blood glucose (Table 1).
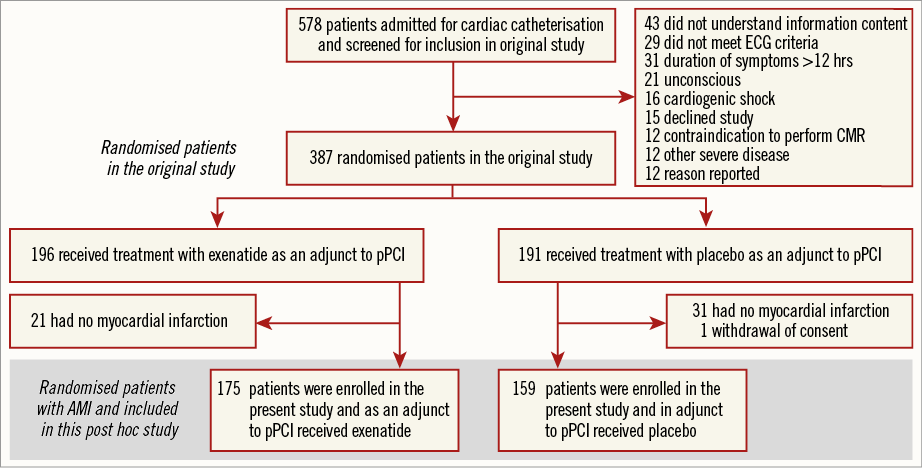
Figure 1. Study flow chart of patients included in this post hoc study. AMI: acute myocardial infarction; CMR: cardiac magnetic resonance; ECG: electrocardiography; pPCI: primary percutaneous coronary intervention
The median follow-up time was 5.2 years (interquartile range: 5.0-5.5). The incidence of the composite endpoint was 85 (25%), with no difference between the treatment groups (42 [24%] in the exenatide group versus 43 [27%] in the placebo group, p=0.44) (Figure 2). The corresponding HR is shown in Figure 3. All-cause mortality was 25 (14%) in the patients treated with exenatide versus 15 (9%) in the placebo group (p=0.20) (Figure 2, Figure 3). Cardiac mortality occurred in nine (5%) and seven (4%) patients in the exenatide and placebo group, respectively (p=0.51). Non-cardiac mortality occurred in 16 (9%) and eight (5%) patients in the exenatide and placebo group, respectively. The incidence of admission for heart failure was significantly lower with 20 (11%) in the group of patients treated with exenatide compared to 31 (19%) in the placebo group (p=0.040) (Figure 2, Figure 3).
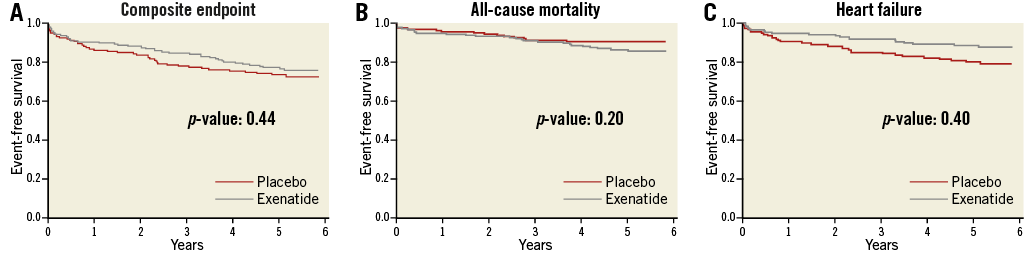
Figure 2. Kaplan-Meier plots of long-term clinical outcome. A) Event-free survival from composite endpoint. B) Event-free survival from all-cause mortality. C) Event-free survival from heart failure.
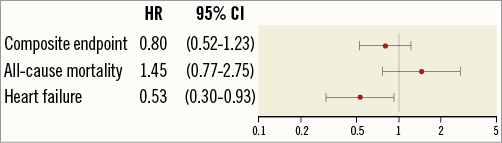
Figure 3. Hazard ratios. Hazard ratio (HR, exenatide vs. placebo) and 95% confidence interval (CI) for the composite endpoint all-cause mortality and heart failure.
Pre-PCI TIMI flow 0/1 was observed in 221 patients. The primary composite endpoint occurred in 65 (29%) of the patients with TIMI 0/1, with no difference between the treatment groups (HR 0.81, 95% CI: 0.54-1.21, p=0.30). Similarly, the all-cause mortality rate did not differ between the groups (HR 1.29, 95% CI: 0.71-2.35, p=0.41). For these patients with pre-PCI TIMI flow 0/1, there was a trend towards a reduction in admission for heart failure (13 [11%] in the exenatide group versus 19 [18%] in the placebo group) (HR 0.59, 95% CI: 0.35-1.00, p=0.05). However, there was no interaction with pre-TIMI flow 0/1 and exenatide treatment in terms of the primary composite endpoint (p for interaction 0.97), all-cause mortality (p for interaction 0.83) or admission for heart failure (p for interaction 0.58).
Discussion
In this post hoc analysis of long-term outcome, exenatide, in addition to standard treatment with primary PCI in STEMI patients, did not reduce the composite endpoint of all-cause mortality and admission for heart failure or all-cause mortality. However, exenatide was associated with a lower incidence of admission for heart failure compared to standard treatment. These findings must be interpreted with caution owing to the small sample size, the post hoc nature of the analysis and the fact that all-cause mortality showed the reverse sign of a non-significant but numerically higher rate in the exenatide group. Nevertheless, these findings are promising and may encourage a larger multicentre study.
In the original study, we found that treatment with the GLP-1 analogue exenatide administered prior to reperfusion by primary PCI increased the myocardial salvage index by 15% in STEMI patients with TIMI flow 0/1 and single-vessel disease7, and that exenatide treatment decreased final infarct size by 30% in STEMI patients with TIMI flow 0/1 and system delay <132 minutes15. Experimental studies in isolated rat hearts and porcine hearts12,13, and clinical studies of exenatide as an adjunct to primary PCI have found similar cardioprotective effects16,17. No large randomised clinical trials testing exenatide or other GLP-1 analogues as cardioprotective agents have been performed; however, improved salvage index and decreased infarct size are associated with improved clinical outcome in patients with STEMI and it might thus be suggested that these compounds could improve clinical outcome22,23. Therefore, our hypothesis in the present study was that an increase in salvage index and a decrease in infarct size observed after exenatide treatment might translate into an improved clinical outcome. We found that exenatide treatment reduced admission for heart failure rates following a STEMI, but did not affect all-cause mortality. However, we observed a numerically higher rate of non-cardiac mortality, but not cardiac mortality. It is known that during the first year after a STEMI mortality is dominated by cardiac causes, after which non-cardiac causes dominate all-cause mortality3. Hence, non-cardiac mortality is not related to the index STEMI and thus probably not to exenatide treatment. Moreover, heart failure should be considered an important endpoint for long-term follow-up of STEMI patients.
In the present study we found an unexpected, numerically higher all-cause mortality rate in patients receiving exenatide. It can be speculated that exenatide provides a certain cardioprotection and decreases cardiac mortality, thereby increasing survival time and thereby the risk of non-cardiac mortality. When we examined causes of death, there was a cardiac mortality of nine (5%) in the exenatide group and seven (4%) in the placebo group and a non-cardiac mortality of 16 (9%) and eight (5%), respectively, which shows that the difference in mortality between the two groups was dominated by non-cardiac mortality. As mortality following a STEMI is mainly due to a cardiac cause within the first year, and thereafter mainly related to non-cardiac causes, it can be assumed that non-cardiac mortality is not related to the index STEMI and thus probably not to exenatide treatment3. It is peculiar that we, in our previous study, demonstrated some cardioprotection from exenatide, but no improvement in left ventricular function in STEMI patients in the original study or this study, but we did observe a reduction in admission for heart failure. However, it could be hypothesised that the cardioprotection exenatide exerted in STEMI patients was small and not detectable in the functional parameters of left ventricular function, but was nevertheless important for the clinical status of patients. It is well known that LVEF is a relatively crude measure for LV function, in part due to compensatory hyperkinesia of the non-infarcted myocardium. Clinical heart failure is associated with adverse outcome despite preserved LVEF24. As ejection fraction is a crude marker of left ventricular dysfunction, small changes in infarct size and myocardial salvage index after a myocardial infarction do not necessarily affect ejection fraction particularly; it could however still affect clinical outcome. Nevertheless, due to the small sample size the risk of a type I error is substantial and this finding must be read with caution and evaluated in a properly powered prospective multicentre study.
Regarding the dose of study medication, we used a continuous intravenous infusion of 25 μg of exenatide commenced before and continued for six hours after PCI in patients with STEMI. The EXAMI study including 39 patients and using a different exenatide treatment regimen of 5 μg loading dose and continuous intravenous infusion of 20 μg/day for 72 hrs was not able to show a significant difference of infarct size16. In the other clinical Exenatide Myocardial Protection in Revascularization Study, Woo et al showed a significant difference in reduction of infarct size and improvement of subclinical left ventricular function after a hybrid administration with a high loading dose of 10 μg subcutaneous injection and 10 μg intravenous bolus before PCI, followed by two days of 10 μg subcutaneous injection twice daily. Our study and treatment algorithm were based on the preclinical work by Sonne et al14. This study demonstrated a bimodal dose-response curve with a wide therapeutic range, since the low dose of 0.03-0.3 nM exendin-4 was cardioprotective, whereas a higher dose of 3.0 nM exendin-4 did not protect the heart during reperfusion. Thus, a larger dose of exenatide would not necessarily translate into a more pronounced effect. In fact, this bimodal response may explain the differences in treatment effect between the studies. The reason for this bimodal response is unknown, but it is very important to take into consideration when designing these kinds of study. The algorithm in our study was constructed in order to have a blood serum concentration of 0.03-0.3 nM, which was protective in the preclinical study. Future studies may focus on finding the optimal effect with the least side effects.
Ischaemic post-conditioning25-27, cyclosporine28, and remote ischaemic conditioning29 have all been shown to be cardioprotective and to reduce reperfusion injury in clinical models, but only remote ischaemic conditioning has been shown to improve long-term clinical outcomes in patients with STEMI undergoing primary PCI30. According to reviews on the clinical studies trying to modify infarct size and clinical outcome, the clinical effects of these treatments have been varying and no studies have shown an evident effect8,10,31. Thus, larger multicentre clinical studies evaluating the effect of cardioprotection on clinical outcome are warranted. Also, there is potential in combining different cardioprotective strategies. However, a potentially cumulative effect of multiple cardioprotection strategies is still untested.
Study limitations
The sample size calculation in the original trial was based on the myocardial salvage index. In the original study patients with multivessel disease or pre-PCI TIMI 2/3 were excluded. These patients were included in the present study to improve the power of the clinical data. Although these patients were included in the present study, our study was not powered to study long-term clinical outcomes, and our findings may represent either a type I or II error. Further, to increase the total number of events, we included ICD/CRT which are not normally considered admission for heart failure events, but we believe that a certain proportion of the ICD/CRT events in this patient population will be heart failure related21.
Conclusion
In this post hoc analysis of STEMI patients treated with primary PCI, additional treatment with exenatide at the time of reperfusion did not reduce the primary composite endpoint or the secondary endpoint of all cause-mortality. However, exenatide treatment may reduce the incidence of admission for heart failure, but this should be confirmed in a larger randomised study.
| Impact on daily practice In STEMI patients with Thrombolysis In Myocardial Infarction (TIMI) flow 0/1 and single-vessel disease, treatment with the GLP-1 analogue exenatide administered prior to reperfusion by primary PCI has been shown to increase the myocardial salvage index. However, treatment with the GLP-1 analogue exenatide administered prior to reperfusion by primary PCI did not show a clear effect on the primary composite endpoint or the secondary endpoint of all cause-mortality. For now, exenatide has not been shown to improve clinical outcome. |
Acknowledgements
The Danish National Research Foundation, Heart Arrhythmia, the Novo Nordisk Foundation, Danielsen’s Foundation, Rigshospitalet’s Research Foundation, and the Danish Heart Foundation supported this research.
Conflict of interest statement
The authors have no conflicts of interest to declare.

