
Abstract
Aims: We aimed to test the hypothesis that the presence of in-stent yellow plaque (YP) assessed by angioscopy would be a risk of very late stent failure (VLSF) of the cobalt-chromium everolimus-eluting stent (CoCr-EES) in comparison with first-generation drug-eluting stents (DES).
Methods and results: DESNOTE-X was a prospective cohort study, an extended study of the DESNOTE study (UMIN000013515). All patients who received successful angioscopic examination at planned one-year follow-up of DES were clinically followed. The primary endpoint was VLSF defined as a composite of cardiac death, target vessel myocardial infarction, and target lesion revascularisation. A total of 504 patients with 549 lesions were enrolled over a period of 12.5 years. At one-year follow-up, the incidence of YP was significantly higher in the first-generation DES than in the CoCr-EES (199/292 [68%] vs 80/257 [31%], p<0.001). Maximum yellow colour grade on coronary angioscopy at one-year follow-up was an independent predictor of future VLSF in the first-generation DES (HR 2.604 [95% CI: 1.265-5.361], p=0.009), whereas it was not in the CoCr-EES (p for interaction 0.022).
Conclusions: The incidence of in-stent atherosclerosis identified as YP on angioscopy was lower and its impact on late clinical events appeared smaller in the CoCr-EES than in the first-generation DES.
Introduction
Although the introduction of drug-eluting stents (DES) substantially reduced early target lesion revascularisation (TLR) as compared to bare metal stents by restricting development of neointimal hyperplasia, DES have been associated with an increased risk of stent thrombosis and TLR after one year, that is, very late stent failure (VLSF)1. In the DESNOTE study in which mainly the first-generation DES was evaluated (86%), neoatherosclerosis identified as an in-stent yellow plaque (YP) on coronary angioscopy one year after the implantation of DES and the absence of statin therapy were risks of VLSF1. The underlying mechanism of VLSF appeared to be the progression of neoatherosclerosis as demonstrated by the YP. However, the incidence of VLSF has been reduced with the use of newer-generation DES2,3,4. Therefore, it would be interesting for physicians to know whether the cause of VLSF of the second-generation DES is also associated with the progression of neoatherosclerosis.
Coronary angioscopy provides substantial information pertaining to macroscopic pathology in living patients. It has reliably detected atherosclerosis as YPs, and especially those with a high-grade yellow colour have been regarded as high-risk plaques and demonstrated to be associated with future coronary events5,6.
The purpose of the present study was to test the hypothesis that the angioscopic findings of the stented segment, especially the presence of in-stent YP, one year after stent implantation would be a risk of future VLSF in the second-generation best-in-class DES, cobalt-chromium everolimus-eluting stent (CoCr-EES) as was demonstrated in first-generation DES.
Methods
STUDY DESIGN AND POPULATION
DESNOTE-X (Detect the Event of very late Stent failure from the angioscopic fiNdings Of drug-eluTing stEnt - XIENCE) was a single-centre prospective cohort study. The present study focusing on the difference between first-generation DES and CoCr-EES was an extended study of the DESNOTE study (UMIN000013515). All patients who received successful angioscopic examination at planned one-year follow-up (±3 months) after DES implantation in the native coronary artery irrespective of clinical presentation (silent myocardial ischaemia, stable or unstable angina, ST-elevation myocardial infarction, or non-ST-elevation myocardial infarction) without any earlier event of stent failure were enrolled from July 2004 to January 2017 and clinically followed up for the occurrence of VLSF. The following DES were defined as first-generation DES: CYPHER® sirolimus-eluting stent (Cordis, Cardinal Health, Milpitas, CA, USA) and TAXUS™ paclitaxel-eluting stent (Boston Scientific, Marlborough, MA, USA). The cobalt-chromium everolimus-eluting stent (CoCr-EES; XIENCE V®, XIENCE PRIME®, XIENCE Xpedition® or XIENCE Alpine™; Abbott Vascular, Santa Clara, CA, USA) was evaluated as a best-in-class second-generation DES. Written informed consent was obtained from all enrolled patients. This study was approved by the Osaka Police Hospital Ethics Committee.
STUDY ENDPOINTS
The primary endpoint of the present study was VLSF, defined as a composite of cardiac death, target vessel myocardial infarction (TVMI), or target lesion revascularisation (TLR)7. The secondary endpoints were all individual components of the primary endpoint and definite or probable stent thrombosis (ST) according to the ARC definition7. An independent clinical events committee adjudicated all clinical events.
PROCEDURE
The PCI strategy was left to the discretion of the individual operators. Dual antiplatelet therapy (DAPT, aspirin and P2Y12 inhibitor) was encouraged for at least one year after the PCI, followed by single antiplatelet therapy with aspirin (100 mg/day). Prolongation of DAPT beyond one year was left to the discretion of the outpatient clinician. All patients and treating physicians were asked to adhere to the Guideline of the Japanese Society of Cardiology in terms of tobacco usage, exercise, healthy food intake, maintenance of an adequate body weight, and medications for the achievement of target blood lipid concentrations, and blood pressure control. One-year invasive follow-up (±3 months) of coronary angiography, mainly via the radial approach, was planned for all patients. Imaging assessment of the stented segment was routinely performed with coronary angioscopy.
ANGIOSCOPIC EXAMINATION
Yellow colour grade, neointimal coverage, and thrombus at the site of DES implantation were examined by angioscopy. The non-occlusion type of angioscopy, VISIBLE (FiberTech Co., Ltd., Tokyo, Japan), was used. Angioscopic observation of the stented lesions was carried out while blood was cleared away from the viewing area by the injection of 3% dextran-408. Cases with complete pullback and good image quality, as defined by >70% of analysable stent length, were included in this analysis9.
Yellow colour grade was classified into four grades (0: white, 1: slight yellow, 2: yellow, and 3: intense yellow) compared with the standard colours6. Neointimal coverage was classified into three grades (0: no coverage, 1: poor coverage, 2: complete coverage)10. Thrombus was defined as white or red material that had a cotton-like or ragged appearance or that presented fragmentation with or without protrusion into the lumen or adherent to the luminal surface11. Image examples are presented in Figure 1. Maximum and minimum neointima coverage grade, maximum yellow colour grade, and the presence or absence of thrombus were determined for each stented lesion. The presence of YP was defined as the maximum yellow colour grade ≥21. Four analysts of angioscopy (Y. Sotomi, S. Suzuki, T. Kobayashi, and Y. Hamanaka) who were blinded to patients’ characteristics evaluated the angioscopic images. The inter-observer and intra-observer reproducibility (percent agreement) for the interpretation of angioscopic images in our institution was 95% and 95% for stent coverage, 85% and 95% for plaque colour, and 90% and 100% for thrombus, respectively1. When a patient had multiple lesions, the lesion with the worst values was chosen as representative for that patient.
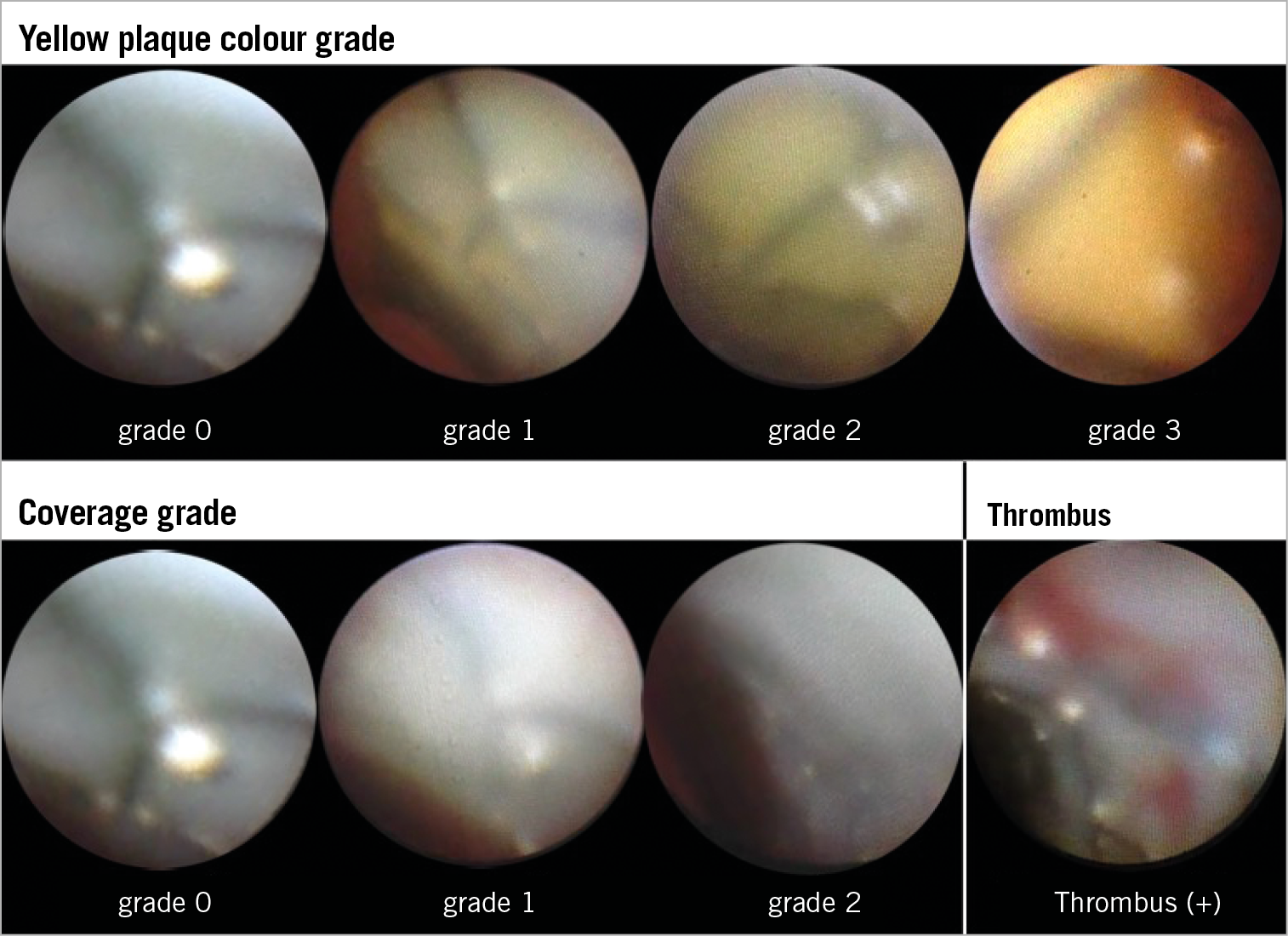
Figure 1. Image examples of coronary angioscopy. Image examples of yellow colour grade, stent coverage grade and thrombus on coronary angioscopy.
STATISTICAL ANALYSIS
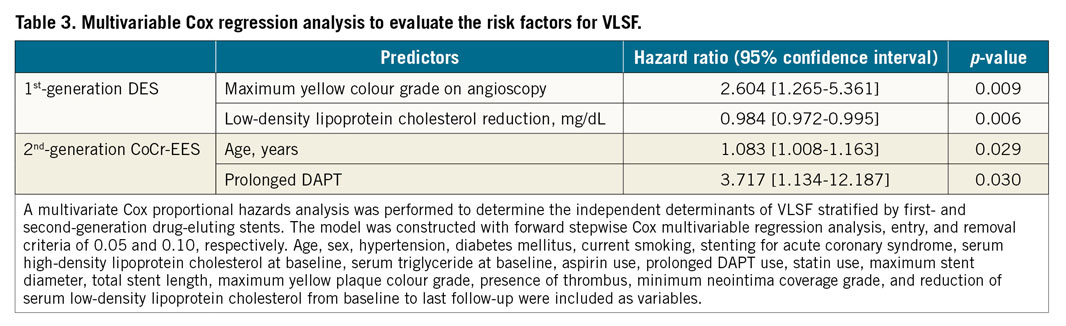
Lesions were divided into four groups according to the presence or absence of YP at the site of stent implantation on coronary angioscopy at one-year follow-up and generation of DES (first-generation DES vs second-generation CoCr-EES). Normality of data distribution was tested by the Kolmogorov-Smirnov test. Data are expressed as mean±SD or median and interquartile range. Group means for continuous variables with normal and non-normal distributions were compared using Student’s t-tests and Mann-Whitney U tests, respectively. Categorical variables were compared using the Pearson’s chi-square test or Fisher’s exact test, as appropriate. A multivariate Cox proportional hazards analysis was performed to determine the independent determinants of VLSF stratified by first-generation DES or second-generation CoCr-EES. The model was constructed with forward stepwise Cox multivariable regression analysis, entry, and removal criteria of 0.05 and 0.10, respectively. Age, sex, hypertension, diabetes mellitus, current smoking, stenting for acute coronary syndrome, serum high-density lipoprotein cholesterol at baseline, serum triglyceride at baseline, aspirin use, prolonged DAPT use defined as continuous DAPT usage beyond one year after index PCI, statin use, maximum stent diameter, total stent length, maximum YP colour grade, presence of thrombus, minimum neointima coverage grade, and reduction of serum low-density lipoprotein cholesterol from baseline to last follow-up were included as variables. The incidence of VLSF was compared between the groups using Kaplan-Meier methods and the log-rank test. A p-value of <0.05 was regarded as statistically significant. A significant level for each paired comparison in the log-rank test was 0.025 after adjustment for multiplicity using the Bonferroni correction. All statistical analyses were performed with SPSS, Version 24.0.0 (IBM Corp., Armonk, NY, USA).
Results
STUDY POPULATION
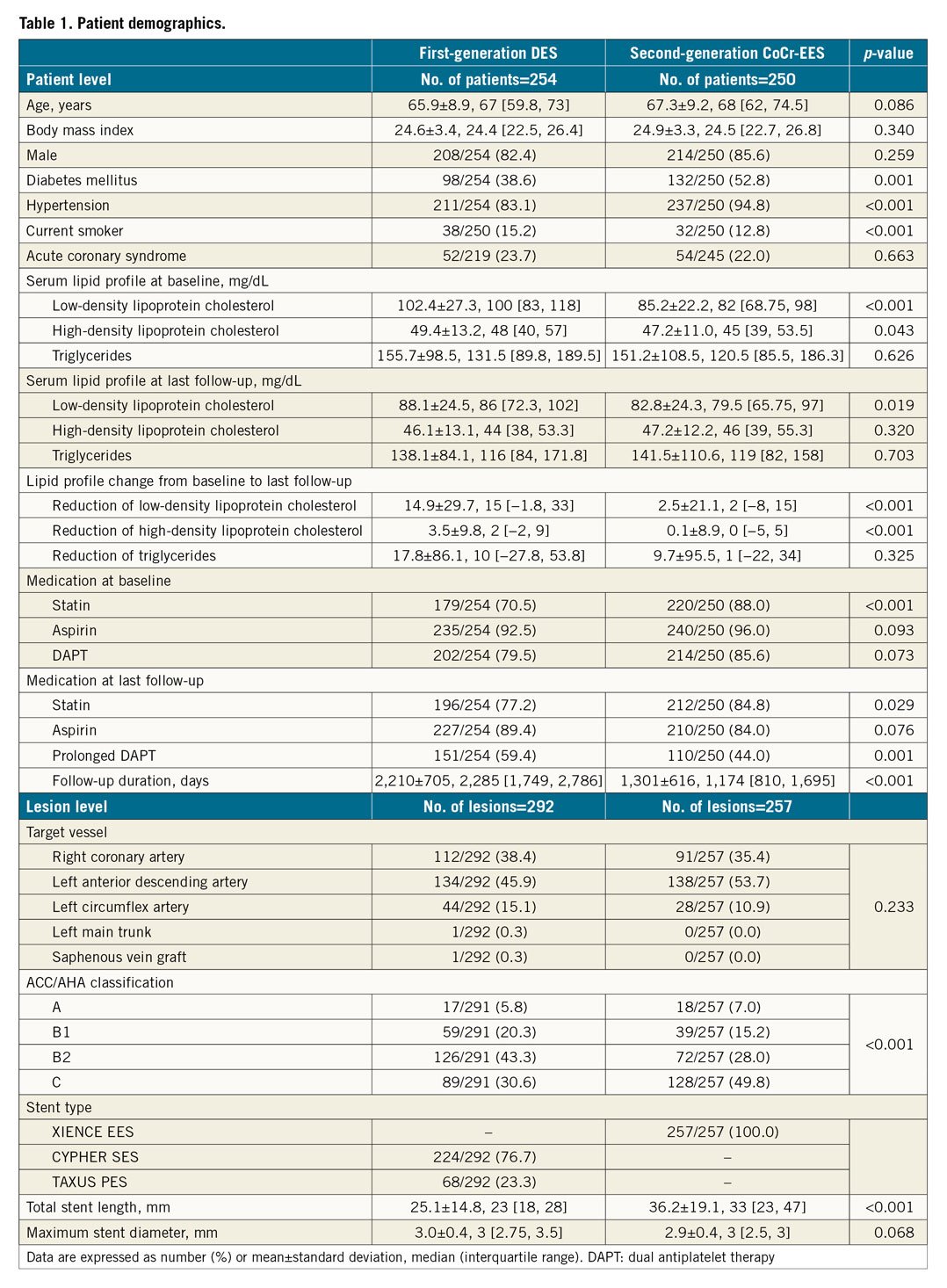
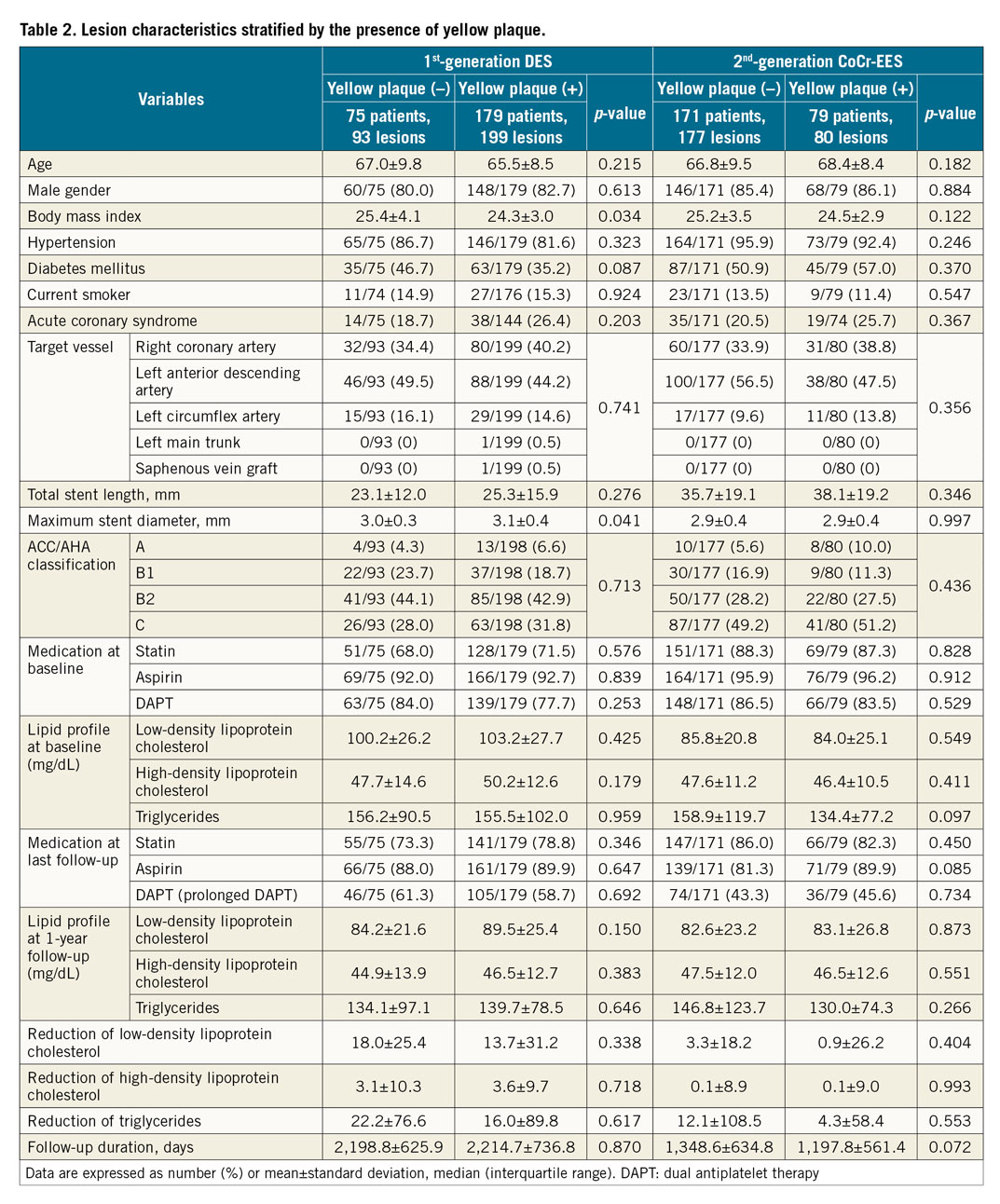
A total of 504 patients with 549 lesions were enrolled over a period of 12.5 years. The median follow-up duration was 1,748 days (interquartile range: 1,079, 2,338 days). Table 1 shows patient and lesion demographics, risk factors, and medication.
ANGIOSCOPIC FINDINGS
Angioscopic findings are shown in Figure 2. At one-year follow-up, the prevalence of in-stent YP was significantly higher in the first-generation DES than in the second-generation CoCr-EES (199/292 [68%] vs 80/257 [31%], p<0.001). In-stent thrombus was also more frequently observed in the first-generation DES than in the CoCr-EES (91/292 [31.2%] vs 57/257 [22.2%], p=0.018). Stent uncoverage (minimum coverage grade=0) was more frequently found in the first-generation DES as compared to the CoCr-EES (153/292 [52.4%] vs 102/257 [39.7%], p=0.002).
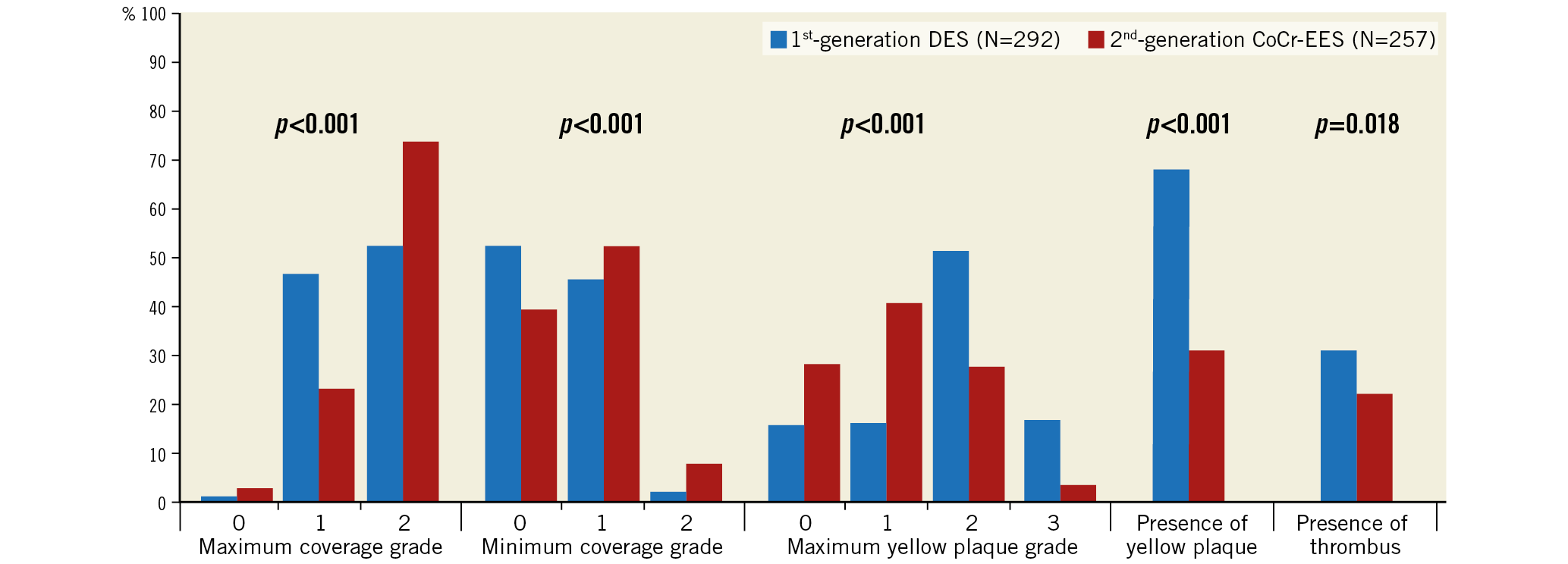
Figure 2. Angioscopic findings. Percentages of each angioscopic finding are presented as bar graphs (chi-square test).
CLINICAL OUTCOMES
Figure 3 and Figure 4 summarise the clinical endpoints. In the first-generation DES, the incidence of VLSF was significantly higher in the YP (+) group than in the YP (–) group (19/199 [9.5%] vs 0/93 [0%], log-rank test p=0.003), which was driven by the higher incidence of TLR in the YP (+) group. In the second-generation CoCr-EES, the incidence of VLSF did not differ significantly between the groups (2/80 [2.5%] vs 11/177 [6.2%], log-rank test p=0.249). Lesion characteristics stratified by the presence of YP in first- and second-generation DES are shown in Table 2.
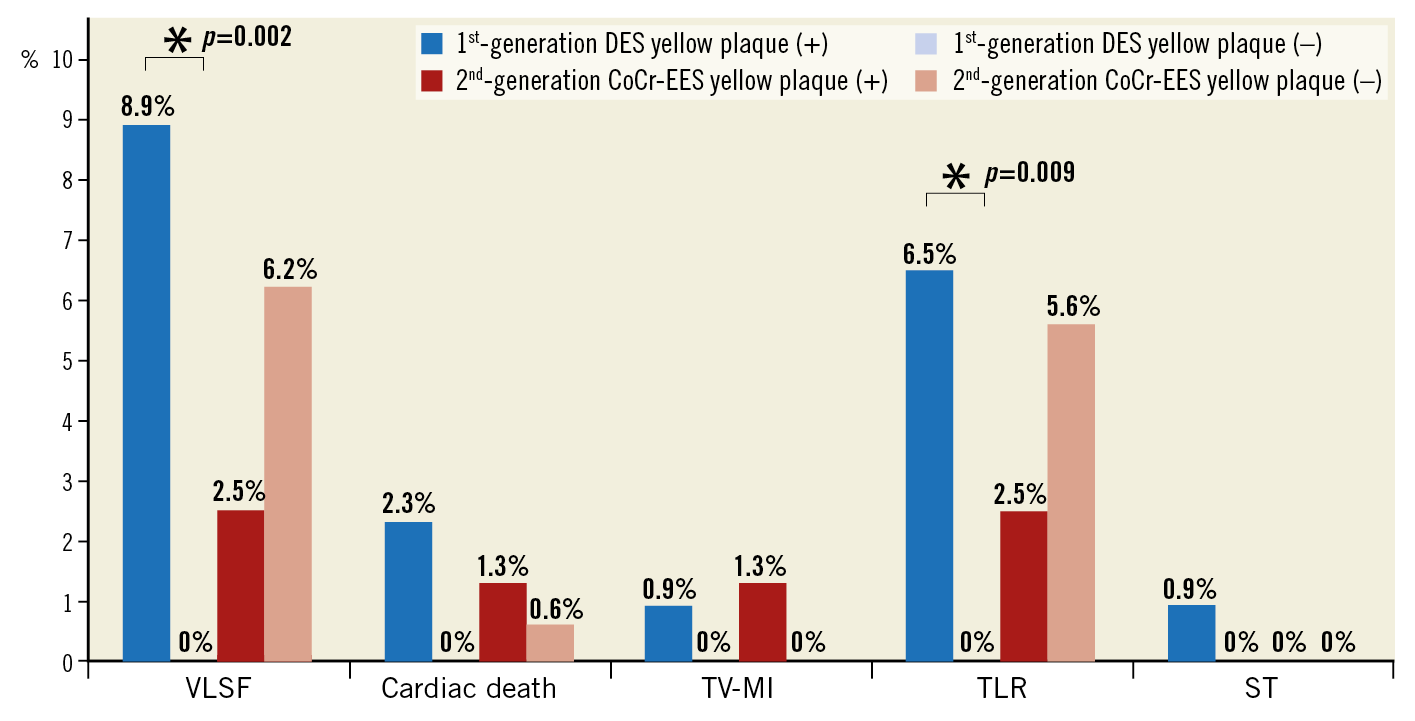
Figure 3. Clinical endpoints. The incidence of the primary and secondary endpoints. *Only p-values <0.05 are presented. The other comparison was not statistically significant. DES: drug-eluting stent; ST: stent thrombosis; TLR: target lesion revascularisation; TV-MI: target vessel myocardial infarction; VLSF: very late stent failure
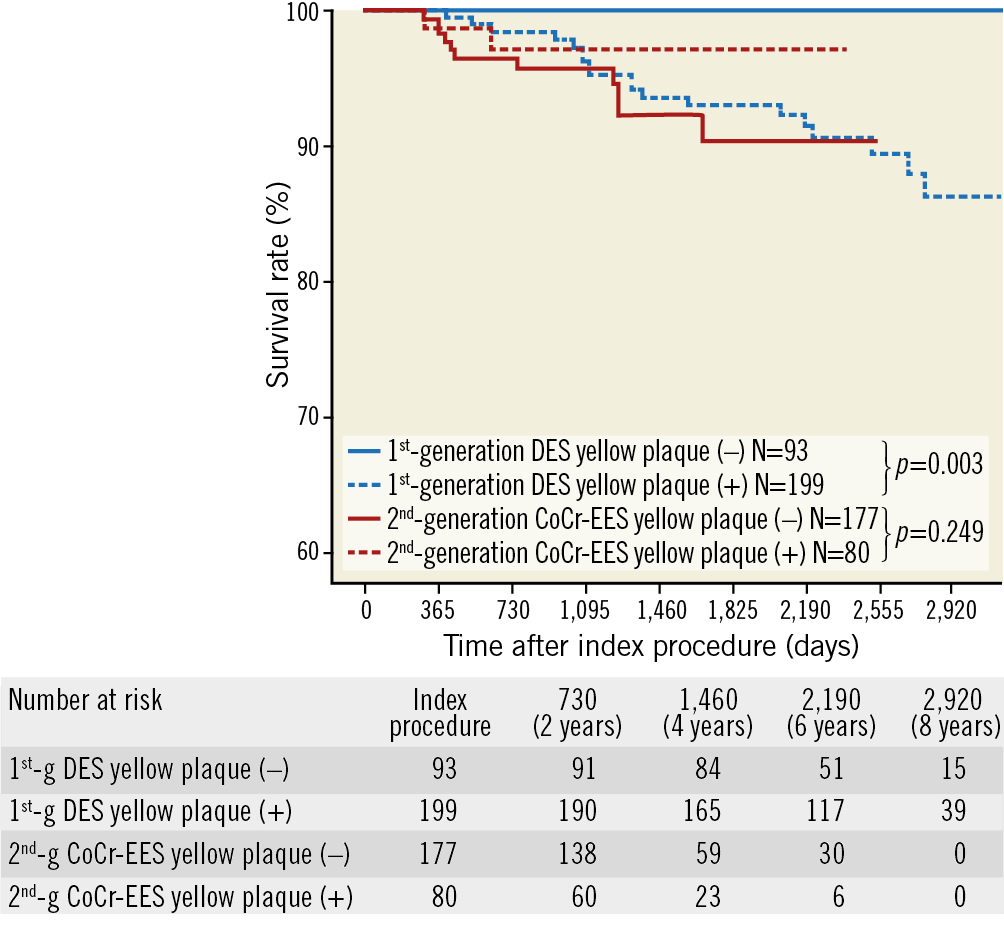
Figure 4. Kaplan-Meier analysis for very late stent failure. Kaplan-Meier analysis for the primary endpoint (very late stent failure, a composite of cardiac death, target vessel myocardial infarction, and target lesion revascularisation) stratified by generation of DES and the presence of yellow plaque on coronary angioscopy
Independent predictors of VLSF in first- and second-generation DES are presented in Table 3. Maximum yellow colour grade on coronary angioscopy at one-year follow-up was an independent predictor of future VLSF in the first-generation DES (HR 2.604 [95% CI: 1.265-5.361], p=0.009), whereas it was not in the CoCr-EES (p for interaction=0.022). In the second-generation CoCr-EES, age and prolonged DAPT were independently associated with late clinical events.
Discussion
The main findings of the present study can be summarised as follows. 1) At one-year follow-up, the incidence of in-stent YP was significantly higher in the first-generation DES than in the second-generation CoCr-EES (p<0.001). 2) In patients without early clinical events after implantation of first-generation DES, VLSF occurred more frequently in the YP (+) group than in the YP (–) group (p=0.003), whereas, in the second-generation CoCr-EES, the incidence of VLSF did not differ significantly between the groups (p=0.249). 3) Yellow plaque colour grade on coronary angioscopy at one-year follow-up was an independent predictor of VLSF in the first-generation DES, whereas it was not in the CoCr-EES.
IMPACT OF IN-STENT YP ON VLSF IN FIRST-GENERATION DES AND SECOND-GENERATION CoCr-EES
Yellow plaque has commonly been detected at the culprit lesions of acute coronary syndrome patients6,12. Yellow plaques, especially those with a high yellow colour grade, are regarded as vulnerable plaques, because they have a high prevalence of disruption-causing thrombus formation and because they have thin fibrous caps12.
Our previous study (the DESNOTE study) demonstrated that in-stent atherosclerosis, evaluated by the presence of YP one year after implantation of DES, was a risk factor for VLSF1. In the DESNOTE study, however, mainly the first-generation DES was evaluated (86%). The second-generation DES can provide better clinical outcomes than the first-generation DES2,3,4,13, which drove us to conduct the current study. In the present study, as compared to the first-generation DES (68%), the prevalence of in-stent YP of the second-generation CoCr-EES XIENCE (31%) decreased to half, despite the fact that the rate of acute coronary syndrome was similar between the groups (22.8% vs 21.5%, p=0.721). This difference observed at follow-up would suggest that a certain part of the in-stent YP was associated with neoatherosclerosis. Progression of neoatherosclerosis might not be so apparent, at least at one-year imaging follow-up, in the CoCr-EES14.
In contrast to the first-generation DES, no significant difference was found in VLSF between patients with in-stent YP and those without (Figure 3, Figure 4). Progression of in-stent atherosclerosis seemed to be strongly associated with VLSF of the first-generation DES, whereas the impact of the progression of in-stent atherosclerosis would seem to be relatively small in the second-generation CoCr-EES. The reason for the negative impact of prolonged DAPT in the second-generation CoCr-EES is a matter of debate. Unlike the DAPT trial, in which myocardial infarction was reduced in the prolonged DAPT arm, TV-MI did not differ in the present study (prolonged DAPT 1/113 [0.9%] vs one-year DAPT 0/144 [0%], p=0.258)15. The negative impact of prolonged DAPT was driven by the high incidence of TLR in the prolonged DAPT patients (prolonged DAPT 9/113 [8.0%] vs one-year DAPT 3/144 [2.1%], p=0.027). It could be speculated that prolonged DAPT might be a surrogate representing physicians’ considerations for high-risk procedures and/or angioscopic findings, and prolonged DAPT itself might not impact negatively on late clinical events. A further prospective large-scale study is warranted to investigate the predictors of VLSF of the newer-generation DES.
NEOATHEROSCLEROSIS FOLLOWING IMPLANTATION OF THE FIRST-GENERATION DES AND SECOND-GENERATION CoCr-EES
At one-year follow-up, the incidence of in-stent YP was significantly higher in the first-generation DES than in the second-generation CoCr-EES. A more modern strategy for dyslipidaemia in patients with CoCr-EES and the difference in lipid profile between groups probably influenced the lower prevalence of atherosclerotic change in neointima among lesions with CoCr-EES. Another possibility could be a device-related mechanism. Incompetent and dysfunctional endothelial coverage of the stented segment could contribute to the neoatherosclerosis16. Poorly formed cell junctions underlie the impaired barrier function of the endothelium, which allows a greater amount of lipoproteins to enter the subendothelial space, leading to the development of neoatherosclerosis17,18. The expression of the platelet endothelial cell adhesion molecule-1 (PECAM-1) was greater in CoCr-EES when compared with the first-generation DES, sirolimus-eluting stent, paclitaxel-eluting stent, and zotarolimus-eluting stent in an animal model19. Therefore, second-generation DES CoCr-EES might not accelerate the formation of YP as compared to the first-generation DES20. The present study would support the validity of this concept in the human coronary artery during very long-term follow-up. On the other hand, human autopsy analysis presented a comparable frequency of neoatherosclerosis in CoCr-EES, sirolimus-eluting stents, and paclitaxel-eluting stents21. The study by Otsuka et al could suffer from the inevitable selection bias due to the principle of being a case control study. However, the study suggested that the main mechanism of the late stent failure could be the same (neoatherosclerosis), in cases where a late clinical event occurs. Progression of neoatherosclerosis might be slower in the second-generation DES than in the first-generation DES, at least up to one-year follow-up. Neoatherosclerosis of the newer-generation biodegradable polymer DES could be the next subject of scientific interest22.
Study strength and limitations
The present study has, to date, the largest study population with the longest follow-up with prospective angioscopic evaluation in patients treated with first-generation DES and the best-in-class second-generation DES, XIENCE CoCr-EES.
However, some limitations of the present study need to be acknowledged. First, although the comparison in the first-generation DES was sufficiently powered, the comparison in the second-generation DES was underpowered in spite of the similar sample size between first- and second-generation DES, presumably due to the unexpected incidence of clinical events. Nevertheless, it is worth noting that significant interaction in the generation of DES existed. The results of the multivariate Cox regression analysis should be interpreted with caution and considered as hypothesis-generating. Second, patients with tortuous or small vessels not suitable for angioscopic examination were excluded, resulting in selection bias. Third, coronary angioscopy could not acquire the images of the whole vessel wall due to the blood flow and might have missed some yellow plaques and thrombus. Finally, although the amount of plaque may impact on VLSF, coronary angioscopy can evaluate only the surface of the plaque and cannot assess the plaque volume precisely.
Conclusions
The incidence of yellow plaque was smaller in the second-generation CoCr-EES than in the first-generation DES. The YP was independently associated with very late clinical events in the first-generation DES, whereas it was not in the second-generation CoCr-EES. The incidence of in-stent atherosclerosis identified as YP on angioscopy was lower and its impact on late clinical events appeared smaller in the CoCr-EES than in the first-generation DES. A further prospective large study is warranted to investigate the underlying mechanisms of the adverse events following newer-generation DES implantation.
|
Impact on daily practice At one-year follow-up, the incidence of yellow plaque was significantly higher in the first-generation DES than in the second-generation CoCr-EES. Maximum yellow colour grade on coronary angioscopy at one-year follow-up was an independent predictor of future stent failure in the first-generation DES, whereas it was not in the second-generation CoCr-EES. Progression of neoatherosclerosis might be slower in the second-generation DES than in the first-generation DES, at least up to one-year follow-up. Neoatherosclerosis of the newer-generation biodegradable polymer DES could be the next subject of scientific interest. |
Conflict of interest statement
Y. Sotomi and S. Nakatani have received speaker honoraria from Abbott Vascular Japan, Boston Scientific Japan, Terumo, Cardinal Health, and Medtronic. Y. Ueda has received a research grant and lecture fee from Abbott Vascular. The other authors have no conflicts of interest to declare.

