
Abstract
Aims: The aim of this study was to compare the long-term outcomes of STEMI patients treated with overlap vs. no-overlap stents.
Methods and results: We analysed the one- and five-year clinical outcomes of 1,498 STEMI patients according to overlapping stent implantation. The primary endpoint was a patient-oriented composite endpoint (PoCE) of all-cause death, myocardial infarction, and repeat revascularisation. Stent thrombosis data were also analysed. Four hundred and four (27.0%) patients were treated with overlapping stents, whereas the remaining 1,094 (73.0%) were not. At one and five years, there was no difference in PoCE between the overlap vs. no-overlap group, even after adjustment (14.9% vs. 12.4%; HR 1.20, 95% CI: 0.76-1.90; p=0.44, and 26.3% vs. 22.3%; HR 1.14, 95% CI: 0.80-1.62; p=0.47, respectively). At five years, within the overlap group, patients who received BMS had a trend towards a higher rate of PoCE and DoCE as compared to those who received EES. At one year, there was a trend towards a higher rate of definite/probable stent thrombosis in the overlap compared to the no-overlap group (2.2% vs. 1.6%; HR 2.35, 95% CI: 0.95-5.90; p=0.06). This difference was driven by a higher rate for BMS compared to EES (4.4% vs. 0%, p for interaction=0.03) in the overlap group. At five years, the absolute risk difference for overlap (3.5% vs. 2.2%, p=0.99) and interaction for BMS (p=0.03) were similar.
Conclusions: In patients presenting with STEMI, the long-term PoCE was similar for the overlap and no-overlap groups. Overlap among patients receiving BMS appears to be associated with a higher risk for adverse cardiovascular outcomes and stent thrombosis. ClinicalTrials.gov number: NCT00828087
Abbreviations
ARC: Academic Research Consortium
BMS: bare metal stent
DES: drug-eluting stent
DoCE: device-oriented composite endpoint
EES: everolimus-eluting stent
HR: hazard ratio
MI: myocardial infarction
PCI: percutaneous coronary intervention
PoCE: patient-oriented composite endpoint
STEMI: ST-segment elevation myocardial infarction
Introduction
In current clinical practice, overlapping stents are reported in up to 30% of patients undergoing percutaneous coronary intervention (PCI). Usually, the most frequent indication for overlapping is long coronary lesions requiring implantation of multiple stents1-3.
In the drug-eluting stent (DES) era, contrasting results have been reported concerning the clinical impact of overlapping stents1-3. In clinical practice, overlapping first-generation DES were associated with impaired clinical outcomes such as death and myocardial infarction (MI)1, whereas data from second-generation DES suggest that overlapping is effective and safe3.
However, most of these findings come from studies enrolling stable coronary artery disease patients and few ST-segment elevation myocardial infarction (STEMI) patients. Moreover, STEMI is a well-known prothrombotic state, where the impact of uncovered or malapposed struts of the overlapping stent may be associated with a high rate of adverse cardiovascular events4.
The objective of the present study was to analyse the clinical impact of overlapping stents in the EXAMINATON trial.
Methods
STUDY DESIGN AND PATIENT POPULATION
The design of the EXAMINATION trial has been described in detail previously5. Briefly, it was a prospective, randomised, multicentre trial that enrolled all-comer STEMI patients. A total of 1,498 patients at 12 centres in three countries were randomised (1:1) to everolimus-eluting stent (EES) (XIENCE® V; Abbott Vascular, Santa Clara, CA, USA) vs. cobalt-chromium bare metal stent (BMS) (MULTI-LINK VISION®; Abbott Vascular) implantation. The study was approved by the institutional review board or ethics committee at each participating centre and all patients signed an informed consent form.
INDEX PROCEDURE AND FOLLOW-UP
At the index procedure, patients received appropriate anticoagulation and other antiplatelet therapies according to standard hospital practice5. Overlap was allowed for long lesions which required two stents or for bail-out situations.
The follow-up included a clinical visit or telephone contacts at 30 days, six months, and one year, and was continued yearly up to five years. No angiographic follow-up was mandated per protocol. All the data were analysed by an independent core lab (Cardialysis BV, Rotterdam, the Netherlands).
OUTCOMES AND DEFINITIONS
This present analysis used the same endpoints as the main EXAMINATION trial, defined according to the Academic Research Consortium (ARC) criteria5,6.
For the purposes of the present study, clinical outcomes were stratified according to the presence of overlapping stents. Endpoints were analysed at either one- or five-year follow-up.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation. Categorical variables are reported as absolute number and percentage. Differences in proportions were tested with the chi-square test or Fisher’s exact test, and differences in continuous variables were tested with a Student’s t-test. The Kaplan-Meier method was used to derive the event rates at follow-up and to plot time-to-event curves. Landmark analysis was performed from zero to one year and from one-year to five-year follow-up.
A Cox proportional hazards model along with a Wald test was used for comparison of outcomes between groups. A crude analysis was performed for all endpoints. A test for interaction between stent type and overlap status was performed for these endpoints: patient-oriented composite endpoint (PoCE), device-oriented composite endpoint (DoCE), and definite/probable stent thrombosis. An adjusted analysis was performed only for PoCE using the following variables: mean age, diabetes mellitus, vasculopathy, left anterior descending artery as target vessel, multivessel disease, use of direct stenting, use of post-dilatation, overlap status, total stent length, mean stent diameter, ST-segment resolution >70%, and EES use. For the assessment of stent failure predictors, defined as DoCE or definite/probable stent thrombosis, all clinical and procedural variables were tested in a univariate Cox model. Those variables with a p<0.05 were introduced into a Cox multivariate model with stent failure as dependent variable.
Results were reported as hazard ratio (HR) with associated 95% confidence interval and p-value. A two-tailed probability value <0.05 was considered significant. All data were processed using the Statistical Package for Social Sciences, Version 22 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CLINICAL CHARACTERISTICS
The overlap group consisted of 404 (27.0%) patients and the no-overlap group of 1,094 patients (73.0%). Baseline patient characteristics are shown in Table 1.
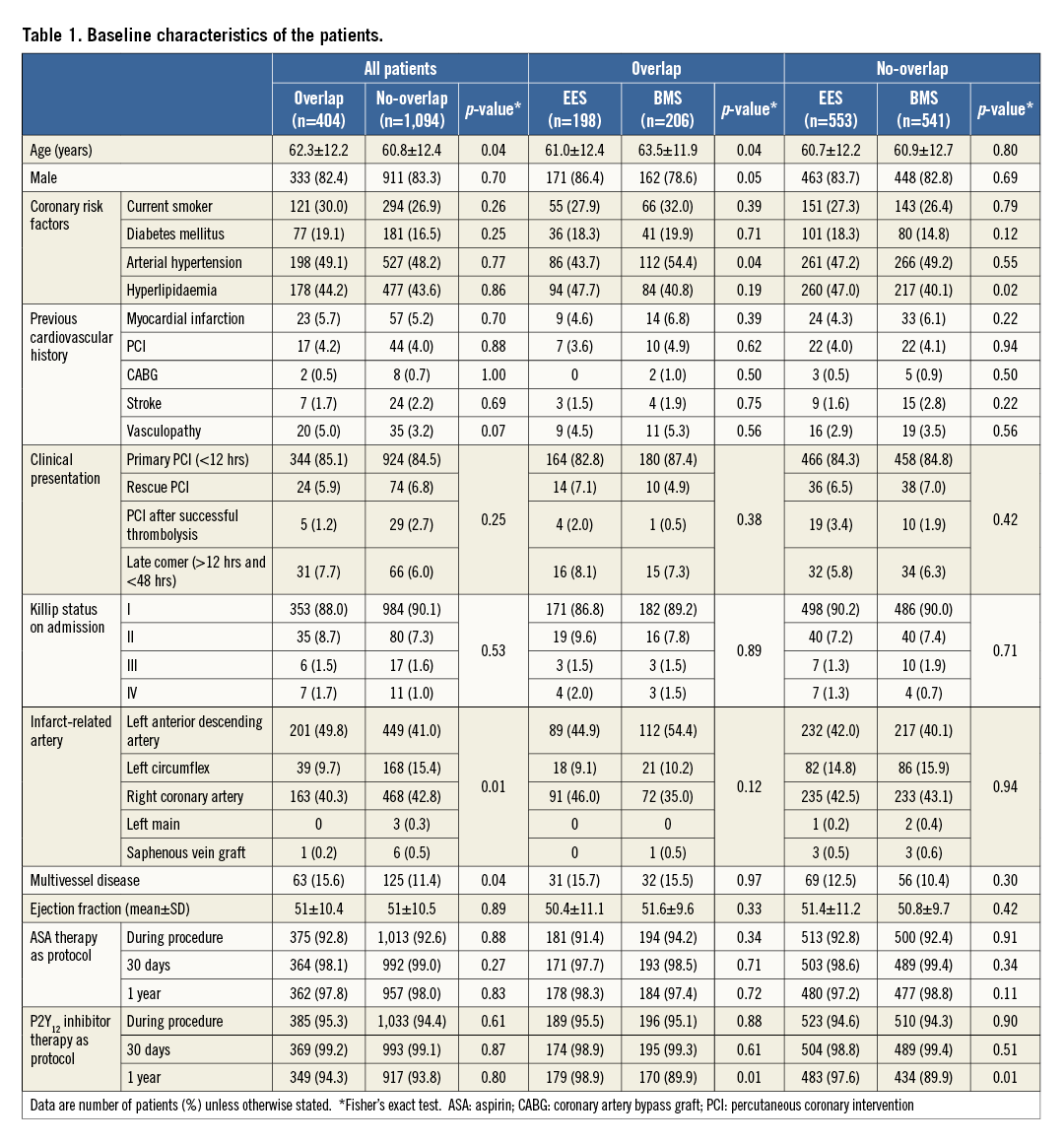
PROCEDURAL CHARACTERISTICS
Procedural characteristics are shown in Table 2. In 84.8% of the cases the reason for implantation of overlapping stents was that the lesion was not completely covered by the first stent (EES: 82.7% and BMS: 87.0%). On the other hand, in 9.3% of the cases the reason for overlapping was coronary dissection (EES: 10.6% and BMS: 7.9%).
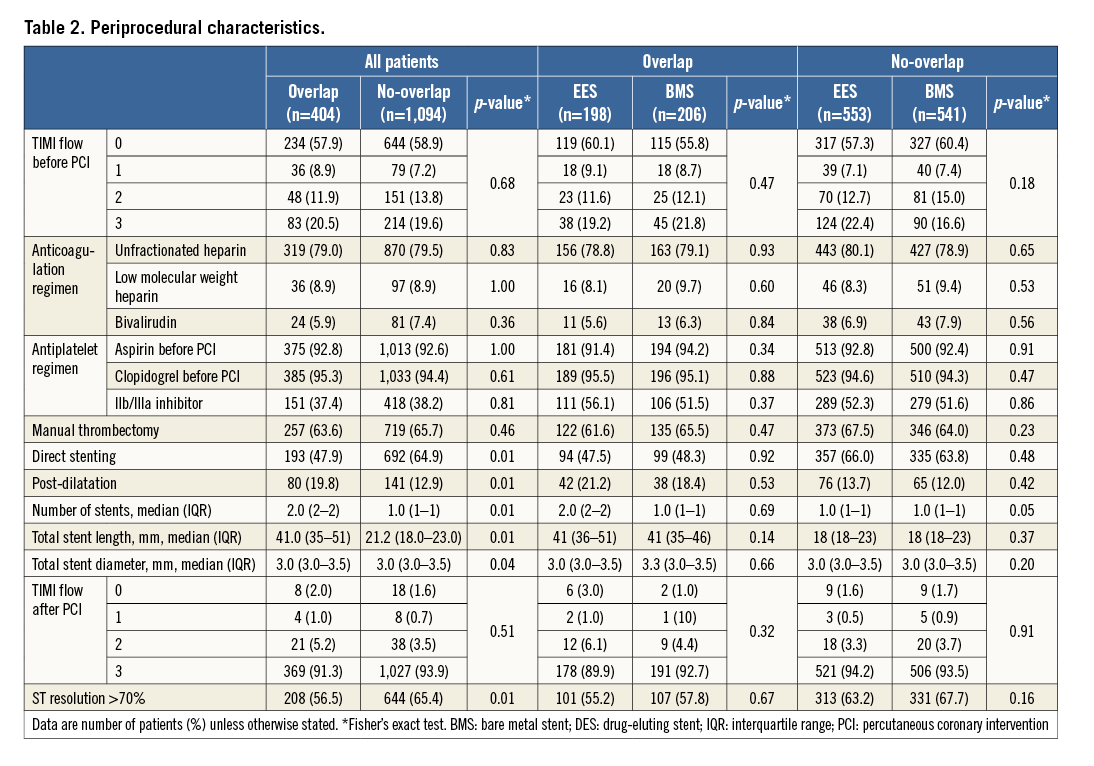
CLINICAL OUTCOMES
ONE-YEAR FOLLOW-UP
At one-year follow-up, there were 196 PoCE events, without difference between the overlap and no-overlap groups (14.9% vs. 12.4%; HR 1.21, 95% CI: 0.89-1.64; p=0.23), even after adjustment (HR 1.20, 95% CI: 0.76-1.90; p=0.44) (Table 3, Figure 1). There were 118 DoCE, without difference between the overlap and no-overlap groups (9.2% vs. 7.4%; HR 1.24, 95% CI: 0.84-1.84; p=0.28) (Table 3, Figure 2).
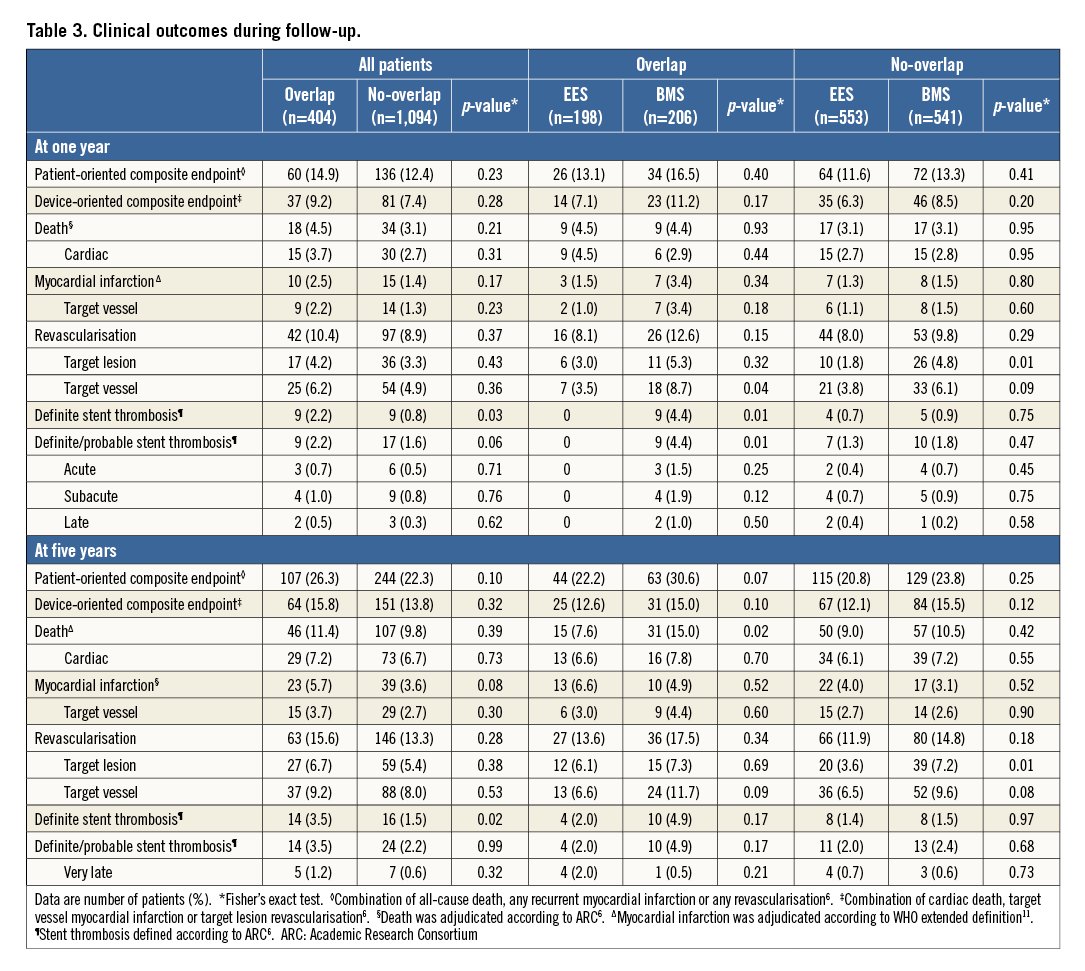
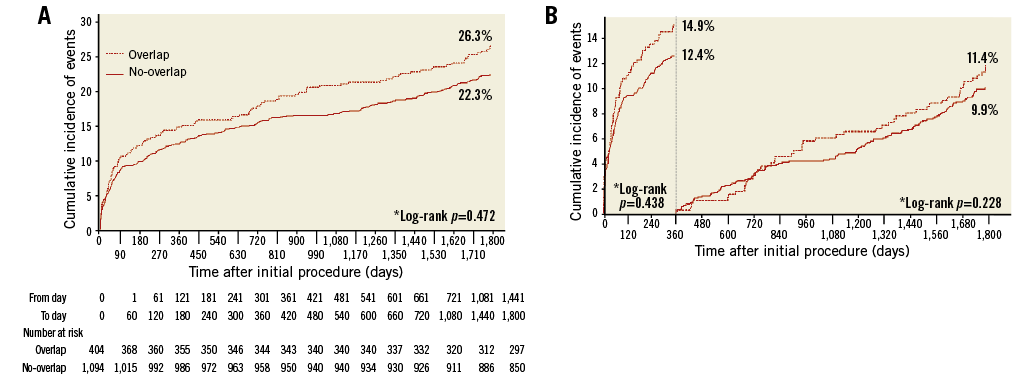
Figure 1. Time-to-event and landmark analyses of PoCE. A) Time-to-event analysis of the patient-oriented endpoint of all-cause death, any myocardial infarction, or any revascularisation over five years between the two groups. B) Landmark analysis for the same endpoint. *Multivariate adjusted
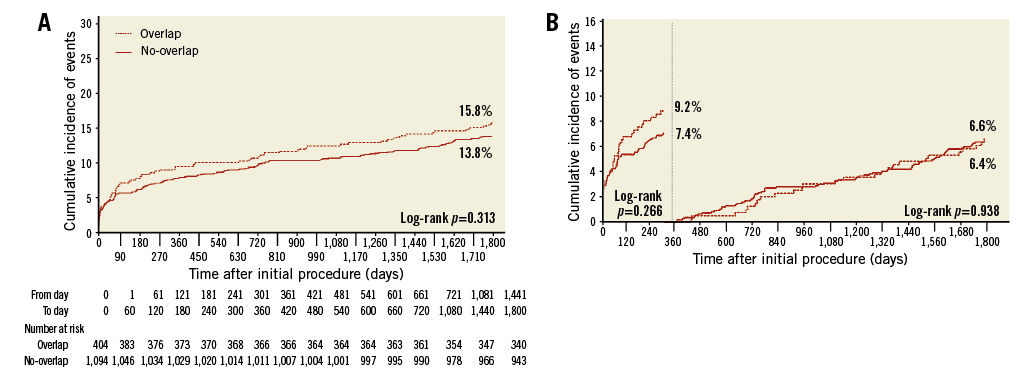
Figure 2. Time-to-event and landmark analyses of DoCE. A) Time-to-event analysis of the device-oriented endpoint of cardiac death, target vessel myocardial infarction, or target lesion revascularisation over five years between the two groups. B) Landmark analysis for the same endpoint.
At one year, within the overlap group, there was no difference according to stent type in terms of PoCE or DoCE (EES: 13.1% vs. BMS: 16.5%; p=0.40, and EES: 7.1% vs. BMS: 11.2%; p=0.17, respectively) (Table 3).
There was a trend towards a higher rate of definite/probable stent thrombosis between the overlap and the no-overlap groups (2.2% vs. 1.6%; HR 2.35, 95% CI: 0.95-5.90; p=0.06, and p for stent type interaction=0.03). There was a lower rate of stent thrombosis in the overlap EES as compared to the overlap BMS group (0% vs. 4.4%; p=0.01) (Figure 3).
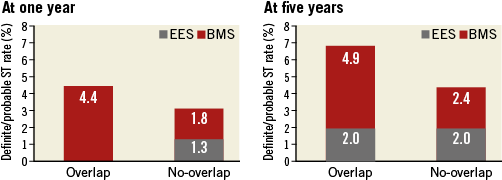
Figure 3. Definite/probable stent thrombosis (def/prob ST) rate in EES and BMS according to overlap status and stent type. At one year, there was a trend towards a higher rate of def/prob ST in the overlap as compared to the no-overlap group (HR 2.35, 95% CI: 0.95-5.90; p=0.06), without difference at five years (HR 1.58, 95% CI: 0.82-3.06; p=0.99). At one- and five-year follow-up, there was a higher rate in the overlap BMS as compared to the overlap EES group (HR 3.36, 95% CI: 1.50-7.53; p=0.01, and HR 2.28, 95% CI: 1.11-4.69; p=0.03, respectively). At one- and five-year follow-up, there was no difference between the no-overlap BMS as compared to the no-overlap EES group (HR 1.11, 95% CI: 0.50-2.44; p=0.80, and HR 1.18, 95% CI: 0.47-1.80; p=0.81, respectively). Furthermore, at one and five years, there was no difference between the overlap EES vs. the no-overlap EES groups (p=0.20 and p=0.98, respectively). Finally, at one and five years, there was a trend towards a higher rate in the overlap BMS compared to the no-overlap BMS group (p=0.07 and p=0.10, respectively).
There was no difference in the definite/probable stent thrombosis rate in the overlap BMS group with ≥23 mm of stent length as compared to the no-overlap BMS group with ≥23 mm (4.4% vs. 3.8%; p=0.78).
FIVE-YEAR FOLLOW-UP
At five-year follow-up, there were 351 PoCE events, without difference between the overlap and no-overlap groups (26.3% vs. 22.3%; HR 1.21, 95% CI: 0.97-1.53; p=0.10), even after adjustment (HR 1.14, 95% CI: 0.80-1.62; p=0.47) (Table 3, Figure 1A). There were 215 DoCE events, without difference between the overlap and no-overlap groups (15.8% vs. 13.8%; HR 1.16, 95% CI: 0.87-1.56; p=0.32) (Table 3, Figure 2A).
At five years, within the overlap group a trend towards a higher rate of PoCE and DoCE was present in the BMS as compared to the EES group (22.2% vs. 30.6%; p=0.07, and 12.6% vs. 15.0%; p=0.10, respectively) (Table 3).
Risk factors for stent failure were age (HR 1.02, 95% CI: 1.01-1.03; p=0.03), previous PCI (HR 2.65, 95% CI: 1.43-4.89; p=0.01), and TIMI flow 0 before PCI (HR 1.48, 95% CI: 1.00-2.22; p=0.05).
There was no difference in the rate of definite/probable stent thrombosis between the overlap and the no-overlap groups (3.5% vs. 2.2%; HR 1.01, 95% CI: 0.32-3.12; p=0.99 and p for stent type interaction=0.03) (Figure 3).
No difference in the definite/probable stent thrombosis rate was found in the overlap BMS group with ≥23 mm of stent length as compared to the no-overlap BMS group with ≥23 mm (4.9% vs. 3.8%; p=0.78).
Landmark analyses did not show differences in terms of PoCE and DoCE at the various time points considered (Figure 1B, Figure 2B).
Discussion
The main findings of our analysis are: 1) in STEMI patients, at one- and five-year follow-up, the overlap group had similar PoCE and DoCE as compared to the no-overlap group; 2) at five-year follow-up, within the overlap group, patients who received BMS had a trend towards a higher rate of PoCE and DoCE as compared to those who received EES; 3) at one-year follow-up, there was a trend towards a higher rate of definite/probable stent thrombosis in the overlap as compared to the no-overlap group.
Previous studies have reported that overlapping of second-generation DES is effective and safe3. However, total stent length as a contributor for adverse patient and device outcomes has also been reported7. The fact that overlapping stents leads to an increased stent length makes it difficult to evaluate the impact of overlap status on clinical outcomes. Furthermore, factors associated with the use of an increased stent length, such as side branch jailing and distal embolisation, were not included in this analysis. Our analysis did not have a sufficient sample size to analyse the impact of stent length on outcomes, so no definite conclusion can be drawn in this regard.
This report extends the observation of previous studies to a high-risk setting, such as STEMI patients1-3. Our results are in line with previous studies, reaffirming that overlapping of newer-generation DES is effective and safe, even in a thrombotic clinical scenario such as STEMI2,3. An additional value of this analysis is that it reports a very long-term follow-up (up to five years), providing an insight into very late events that have previously been evaluated only up to three-year follow-up2,3.
Of note, within the overlap group, at long-term follow-up there was a trend towards a higher rate of PoCE and DoCE in the BMS group, with an increase in all-cause death and target vessel revascularisation, without difference in cardiac death and target lesion revascularisation. Most of these results resemble the main findings of the EXAMINATION trial and highlight the superiority of EES over BMS8.
We found a trend towards a higher rate of definite/probable stent thrombosis at one-year follow-up in the overlap as compared to the no-overlap group, with a higher rate in patients receiving BMS as compared to those who received EES. These findings expand the observation of a higher thrombosis of BMS as compared to EES in STEMI patients9, identifying a possible special procedural context of an even higher risk, which consists of stent overlap. However, at five-year follow-up, no difference was found either according to overlap status or by stent type. As this is a post hoc analysis, these findings should be considered as hypothesis-generating.
Finally, stent restenosis in the BMS group could have been an important contributor to long-term PoCE and DoCE. Additionally, the development of in-stent restenosis leading to a subsequent stent thrombosis could be a mechanistic explanation related to the late and very late events10.
Limitations
This analysis has several limitations. First, this was a post hoc analysis and overlap status was not a pre-specified subgroup analysis in the EXAMINATION trial. Second, the trial was not powered for low rate events such as mortality or stent thrombosis. Third, there were baseline differences between the overlap and no-overlap groups. Despite these differences, an adequate statistical treatment was performed to minimise their impact on the results. Fourth, the SYNTAX score was not calculated. Fifth, at the time this trial was conducted, neither prasugrel nor ticagrelor was approved for use in clinical practice, thus all patients were treated with clopidogrel. Despite these limitations, this analysis also has important strengths such as being an all-comers study, analysis of relevant clinical and device endpoints, and the longest available follow-up. These strengths may provide a scenario similar to “real-world” clinical practice.
Conclusions
In patients presenting with STEMI and undergoing PCI, the long-term PoCE was similar in the overlap as compared to the no-overlap group, even after adjustment for confounding variables. Overlap among patients receiving BMS appears to be associated with a higher risk for adverse cardiovascular outcomes and stent thrombosis.
| Impact on daily practice In patients presenting with STEMI and undergoing PCI, the long-term PoCE was similar in the overlap as compared to the no-overlap group, even after adjustment for confounding variables. Overlap among patients receiving BMS appears to be associated with a higher risk for adverse cardiovascular outcomes and stent thrombosis. In a STEMI scenario, if overlapping stents are needed, the use of newer-generation DES should be considered over BMS. |
Guest Editor
This paper was guest edited by Donald Cutlip, MD; Cardiac Catheterization Laboratory, Beth Israel Deaconess Medical Center, and Harvard Medical School, Boston, MA, USA.
Funding
Spanish Heart Foundation.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor declares receiving research support paid to the institution from Boston Scientific and CeloNova.

