Abstract
Aims: We sought to determine the impact of post-dilation (PD) on clinical outcomes in a large cohort of patients treated only with the Absorb Bioresorbable Vascular Scaffold (BVS).
Methods and results: We evaluated all consecutive patients enrolled in the multicentre, single-arm ABSORB EXTEND study up to June 2013. The study allowed treatment of up to two coronaries (diameter 2.0 to 3.8 mm) and the use of overlapping (lesion length ≤28 mm). Patients with severe lesion calcification/tortuosity were excluded. Aggressive lesion predilation (balloon to artery ratio of 0.9-1.0) was mandatory, and PD was left to the operator’s discretion. Patients were grouped according to whether PD was performed or not, and the one-year incidences of MACE and scaffold thrombosis were compared. A total of 768 patients were enrolled in the study; PD was performed in 526 (68.4%). There were no significant differences between the PD group and non-PD group in the majority of baseline characteristics, including the presence of moderate calcification and of B2/C lesions. Lesion length was similar (12.3±5.1 mm vs. 12.1±5.3 mm, p=0.6), as was RVD (2.6 mm for both groups, p=0.2). Residual in-scaffold stenosis (15.5±6.4% with PD, 15.0±6% without PD, p=0.3) and the need for bail-out scaffold/stent (4.2% with PD, 4.6% without PD, p=0.8) were comparable. Acute gain was higher in the non-PD group (1.14±0.3 mm vs. 1.21±0.4 mm, p=0.02). Clinical device success was 98.9% in both groups. At one year, there was no difference in MACE (5.4% in the PD group vs. 2.5% in the non-PD group, p=0.1). All individual components of TLR, death, and MI were similar as well as definite/probable scaffold thrombosis between the two groups.
Conclusions: These results reflect very similar final angiographic and clinical results achieved with or without post-dilation in the treatment of low to moderately complex coronary lesions. Therefore, post-dilation should be performed whenever needed to optimise acute results.
Abbreviations
BRS: bioresorbable scaffold(s)
DES: drug-eluting stent(s)
EES: everolimus-eluting stent(s)
ID-TLR: ischaemia-driven target lesion revascularisation
IVUS: intravascular ultrasound
MI: myocardial infarction
OCT: optical coherence tomography
PLLA: poly-L-lactide
RVD: reference vessel diameter
SDV: source document verification
TIMI: Thrombolysis In Myocardial Infarction
Introduction
In 1995, Colombo et al, using high-pressure final balloon dilations guided by intravascular ultrasound (IVUS), showed that the metallic Palmaz-Schatz stent could be safely deployed, with reduced acute/subacute stent thrombosis and without the use of anticoagulation, provided that stent expansion was adequate with no residual flow-limiting stenosis1. In that seminal publication, the authors set the standard for the deployment technique for contemporary metallic stents.
Following this, many subsequent publications confirmed that both stent thrombosis and restenosis rates were related to final stent dimensions in both bare metal and drug-eluting stents2-6.
More recently, bioresorbable scaffolds (BRS) have been developed as an attractive alternative to metallic stents as the need for mechanical support for the healing artery is temporary, and beyond the first few months there are potential disadvantages of a permanent metallic prosthesis. However, due to intrinsic properties related to its design and composition, the deployment of BRS requires a more aggressive lesion preparation. The role of high-pressure final post-dilation still remains unclear.
We sought to determine the impact of post-dilation on clinical outcomes of a large cohort of patients treated solely with the Absorb Bioresorbable Vascular Scaffold (BVS) system (Abbott Vascular, Santa Clara, CA, USA).
Methods
STUDY DESIGN AND POPULATION
For the current study we evaluated all consecutive patients enrolled in the multicentre, single-arm ABSORB EXTEND study up to June 2013. Patients were grouped according to whether post-dilation was performed or not, and the cohorts were compared in terms of acute and up to one-year clinical outcomes.
Details of the EXTEND study have been previously published7. In brief, the EXTEND study is a prospective, single-arm, open-label clinical study that was planned to register approximately 800 patients at up to 60 sites outside the USA. Patients were eligible if they were ≥18 years with evidence of myocardial ischaemia (e.g., stable or unstable angina, silent ischaemia, positive functional study or a reversible change in the 12-lead electrocardiogram [ECG] consistent with ischaemia). Target vessels should have a reference vessel diameter (RVD) ≥2.0 mm and ≤3.8 mm with a maximum lesion length of ≤28 mm, a percentage diameter stenosis ≥50% and <100% and a Thrombolysis In Myocardial Infarction (TIMI) flow grade of ≥1. A maximum of two de novo native coronary artery lesions could be treated, each located in a different major epicardial vessel. Major exclusion criteria included presentation with recent myocardial infarction (<72 hours to the index procedure), target lesions located in the left main or within an arterial or saphenous vein graft. Also excluded were lesions with excessive tortuosity and/or heavy calcification.
Source document verification (SDV) was routinely performed in 100% of all reported events and 100% of patients up to 30-day follow-up. Subsequently, SDV was performed in a random 20% of patients for the remaining follow-up visits.
Assessment of angina status, data collection of adverse events, details of any subsequent coronary interventions, and use and changes in concomitant medications were collected at 30 days (±7 days), 180 days (±14 days) and one, two and three years (±28 days), by dedicated research staff, following a pre-specified questionnaire. Invasive follow-up was not part of the ABSORB EXTEND protocol, and therefore repeat angiography was only performed based on the presence of ischaemia and/or recurrence of angina symptoms.
The ABSORB EXTEND study is sponsored and funded by Abbott Vascular. The research ethics committee of each participating institution approved the protocol and all enrolled patients provided written informed consent before inclusion. The study is registered at ClinicalTrials.gov (unique identifier: NCT01023789).
STUDY DEVICE
The study device (Absorb BVS) is the same as used for the ABSORB cohort B trial, and has been described in detail previously5,6. In brief, the balloon-expandable Absorb BVS comprises a poly-L-lactide (PLLA) backbone, coated with a matrix composed of the antiproliferative drug everolimus (Novartis Pharmaceuticals Corporation, Basel, Switzerland) and polymer poly(D,L-lactide) (PDLLA) in a 1:1 ratio to form an amorphous drug-eluting coating matrix containing 100 µm everolimus/cm2. Both PLLA and PDLLA are fully bioresorbable: PDLLA is expected to be totally resorbed by the body in nine months and PLLA in approximately 36 months. Its current strut thickness is 158 μm and the crossing profile is about 1.4 mm.
STUDY PROCEDURE
Patients were registered through an interactive voice response system (Oracle America, Inc., Woburn, MA, USA) following confirmation of angiographic inclusion criteria and delivery of the Absorb device beyond the guiding catheter.
All target lesions were to be treated using “aggressive” predilation (balloon to artery ratio of 0.9 to 1.0) and scaffold implantation at a pressure not exceeding the rated burst pressure.
Post-dilation was left to the discretion of the investigator. However, if performed, a non-compliant balloon, sized to fit within the expansion limits of the scaffold, had to be used. Importantly, the post-dilation balloon size should not exceed the nominal diameter of the implanted scaffold by more than 0.5 mm (e.g., a 3.0 mm Absorb BVS could be post-dilated with a maximum 3.5 mm non-compliant balloon, up to its nominal pressure).
It was not recommended to reintroduce an unexpanded scaffold into the artery once pulled back into the guiding catheter. In the event of bail-out and additional stent/scaffold requirement, a commercially available EES or an Absorb system was recommended.
All patients enrolled in the study were to be pre-treated with a loading dose of 300-600 mg of clopidogrel and ≥300 mg of aspirin, followed by 75 mg of clopidogrel daily for a minimum of six months and ≥75 mg of aspirin for the length of the clinical investigation.
Study endpoints and definitions
The main goal of the present analysis was to compare the incidence of in-hospital and one-year major adverse cardiac event (MACE) and scaffold thrombosis rates following Absorb BVS implantation between patients treated with and without post-dilation.
Additionally, by means of quantitative coronary angiography (QCA) measurements, we sought to compare the acute impact of post-dilation on the angiographic performance of this BRS.
MACE comprised cardiac death, myocardial infarction (MI) and ischaemia-driven target lesion revascularisation (ID-TLR).
Cardiac death was defined as any death due to proximate cardiac cause (e.g., MI, low-output heart failure, fatal arrhythmia). Unwitnessed death and deaths of unknown cause were classified as cardiac death. This included all procedure-related deaths including those related to concomitant treatment.
MI classification and criteria for diagnosis were defined according to the per protocol definition: Q-wave MI (QMI) was the development of a new, pathological Q-wave while non-QMI was elevation of CK levels to ≥2 times the upper limit of normal with elevated CK-MB in the absence of new pathological Q-waves.
ID-TLR was defined as any repeat percutaneous intervention of the target lesion or bypass surgery of the target vessel with either positive functional ischaemia study, ischaemic symptoms and angiographic minimal lumen diameter stenosis ≥50% by core laboratory QCA, or revascularisation of a target lesion with diameter stenosis ≥70% by core laboratory QCA without either ischaemic symptoms or a positive functional study.
Scaffold thrombosis was categorised as acute (<1 day), subacute (1-30 days) and late (>30 days) and was defined according to the ARC definition (definite: acute coronary syndrome and angiographic or pathologic confirmation of scaffold thrombosis; probable: unexplained death ≤30 days or TV-MI without angiographic information)8.
Device success was defined as successful delivery and deployment of the clinical investigation scaffold at the target lesion and successful withdrawal of the scaffold delivery system with attainment of final residual stenosis <30% by QCA (by visual estimation if QCA was unavailable).
Clinical success was defined as successful delivery and deployment of the clinical investigational scaffold at the target lesion and successful withdrawal of the scaffold delivery system with attainment of final residual stenosis of <30% by QCA (by visual estimation if QCA unavailable) and/or using any adjunctive device without the occurrence of ischaemia-driven major adverse cardiac events (MACE) during the hospital stay with a maximum of the first seven days post index procedure. In a dual lesion setting both lesions must have met clinical procedure success.
QCA was performed by an independent core lab (Cardialysis, Rotterdam, The Netherlands). All study endpoint events were adjudicated by an independent clinical events committee according to protocol definitions and/or the Academic Research Consortium (ARC) definitions. All adverse events were reported to an independent data and safety monitoring board, which reviewed the data to identify any safety issues related to the conduct of the study.
STATISTICAL ANALYSIS
For the descriptive statistics, categorical data are presented as counts and percentages and continuous variables are presented as mean±standard deviation (SD). Categorical variables were compared by use of the chi-square test or the Fisher’s test when the Cochran’s rule was not met. Continuous variables were compared by the Student’s t-test. To determine the independent predictors of MACE, a multivariable logistic regression model was built using a stepwise (forward/backward) procedure, with independent variables entered into the model at the 0.20 significance level and removed at the 0.10 level. Variables were eligible for inclusion in the multivariable logistic regression model-building process if they had a p-value <0.1 from the univariable analysis.
A two-tailed p-value <0.05 was considered statistically significant.
Results
BASELINE CLINICAL AND ANGIOGRAPHIC CHARACTERISTICS
A total of 768 patients (826 lesions) were included in the present analysis. Post-dilation was performed in the majority of the cases (n=526 patients, 68.4%). The mean population age was 61 years, with more than 70% of the patients being male in both groups. The two cohorts did not significantly differ with regard to main baseline clinical characteristics, with the exception of a higher prevalence of dyslipidaemia among those who underwent post-dilation (74.9% vs. 64.5%, p=0.003) and the presence of prior CVA or stroke (4.6% vs. 1.2%, p=0.02). Table 1 details the baseline clinical profiles of the two groups.
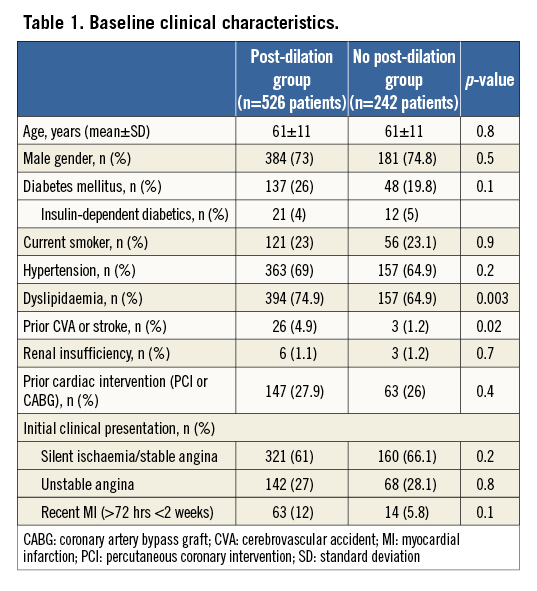
Table 2 displays the main pre-intervention angiographic findings. Of note, the vast majority of the individuals in both groups underwent single-vessel PCI (92.0% in the group with post-dilation vs. 93% in the cohort without post-dilation, p=0.6). The left anterior descending (LAD) coronary artery was the most frequently treated coronary in both cohorts, corresponding to roughly half of the target vessels. Although not allowed by protocol, the left main stem was treated with Absorb BVS in both groups (0.9% in the cohort with post-dilation and 0.4% in the group without post-dilation, p=0.7).
In addition, the main pre-intervention lesion characteristics did not significantly differ between the two groups, including lesion angulation >45° (2.5% vs. 3.9% for patients with and without post-dilation, respectively, p=0.2) and presence of moderate/severe lesion calcification (13.7% in the group with post-dilation vs. 12.7% in the group without, p=0.7).
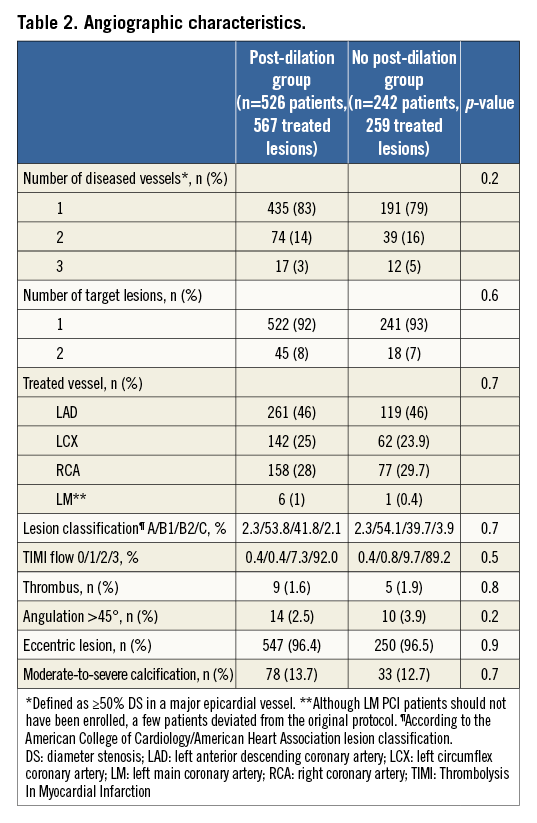
QCA FINDINGS
Table 3 summarises the main pre- and post-intervention QCA findings. Pre-procedure lesion length was relatively short in both groups (12.3±5.1 mm vs. 12.1±5.3 mm, p=0.6). Regarding vessel sizing, as recommended by protocol, scaffold size was selected based on Dmax measures which also did not significantly differ between the groups, either in the proximal (2.85±0.39 mm vs. 2.85±0.37 mm, p=0.8) or distal (2.72±0.39 mm vs. 2.68±0.37 mm, p=0.2) treated segments. In the PD group, pre-intervention MLD was slightly but significantly smaller and % diameter stenosis was modestly but significantly lower than in the non-PD group (1.13±0.32 mm vs. 1.05±0.32 mm, p=0.002, and 57.4±10.0% vs. 59.3±11.7%, p=0.03, respectively). Scaffold deployment pressure was similar in both groups (13.2±2.7 atm for the PD cohort vs. 14.3±1.9 atm, p=0.4). In the group submitted to PD, the final post-dilation pressure was 16.7±3.6 atm. Final balloon dimensions were slightly greater in the PD group (3.3±0.3 mm vs. 2.9±0.4 mm in the control group, p=0.03).

At the end of the procedure, in-scaffold acute gain was higher among those without post-dilation (1.14±0.3 mm vs. 1.21±0.4 mm, p=0.02) but with equivalent in-scaffold minimum lumen diameter (2.28±0.31 mm vs. 2.26±0.28 mm, p=0.4) and residual stenosis (15.5±6.4% vs. 15.0±6.1%, p=0.3) in the groups with and without post-dilation, respectively.
Notably, among patients in whom scaffold overlapping was planned, post-dilation was more often performed (12.2% vs. 7.0%, p=0.03). The mean length of scaffolds implanted was longer in the PD group (24.0±10.3 mm vs. 21.8±8.3 mm, p=0.001).
Of note, post-dilation did not result in increased need of bail-out scaffold/stent (4.2% of bail-out among those with post-dilation vs. 4.5% among those without post-dilation, p=0.8).
IN-HOSPITAL AND ONE-YEAR CLINICAL OUTCOMES
Although the rate of clinical device success was similar in both groups (98.9% vs. 98.8%, p=1.0), clinical procedure success was significantly higher among those without post-dilation (98.8% vs. 96.2%, p=0.05). This finding was due exclusively to an increase in protocol-defined periprocedural non-Q-wave MI in patients who received post-dilation (2.7% vs. 0, p=0.007). During the in-hospital phase there were no cardiac deaths, Q-wave MI or need for additional urgent TLR in either cohort.
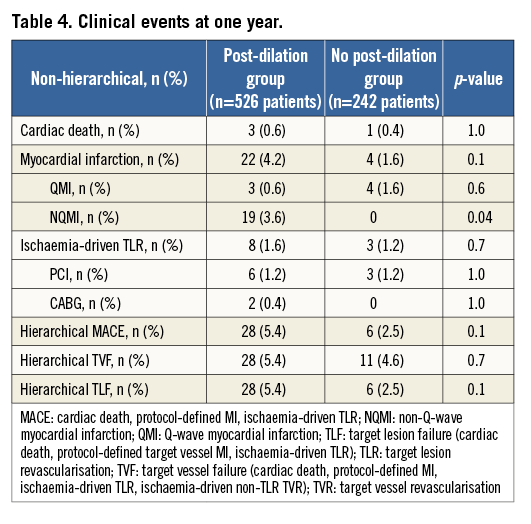
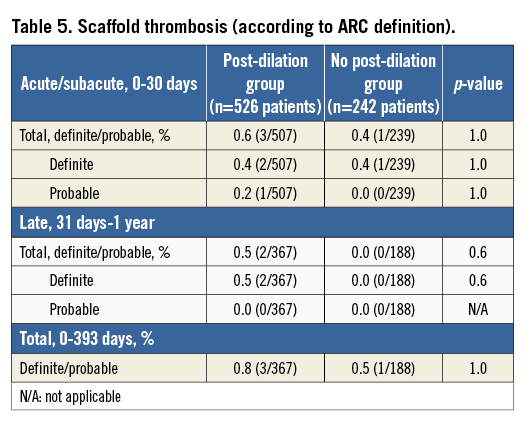
At one year, the MACE rates for patients with and without post-dilation were 5.4% vs. 2.5%, p=0.1, respectively (Table 4, Table 5). The rates of ID-TLR and definite/probable scaffold thrombosis for the two populations were not different (1.6% vs. 1.2%, p=0.7, and 0.6% vs. 0.4%, p=1.0, respectively). As previously mentioned, the major difference in the MACE rate between the two groups was a result of the marginally higher rate of periprocedural non-Q-wave MI in patients with post-dilation. TLR and scaffold thrombosis rates were comparable at each time point. The cumulative cardiac death rate was very low for both cohorts (about 0.5% at one year). Figure 1 shows the curves for cumulative MACE, MI and definite/probable stent thrombosis up to one year of clinical follow-up.
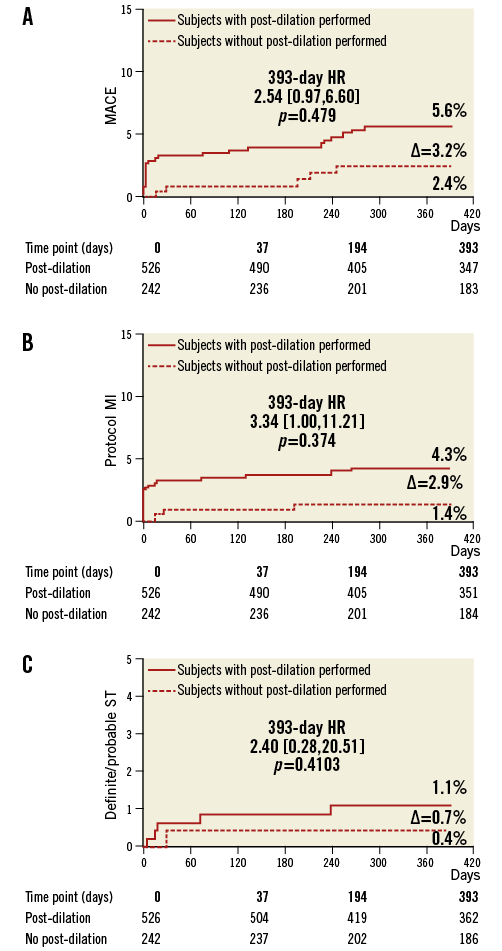
Figure 1. Cumulative incidence of combined MACE (A), MI (B) and scaffold thrombosis (C) in the ABSORB EXTEND registry. Most of the MI in the PD cohort occurred in the in-hospital period (periprocedural MI). This finding drove the MACE difference between the two groups, also more evident in the in-hospital phase.
There was one case only of definite subacute scaffold thrombosis in the cohort without post-dilation. At the index procedure, an Absorb BVS (3.0×18 mm) was implanted in the mid LAD without procedural complications. The patient presented with an acute MI related to the target vessel on day 29 post procedure. Diagnostic angiography revealed a core lab-assessed in-scaffold thrombosis with 23% diameter stenosis of the mid LAD. Of note, aspirin and clopidogrel were stopped on day 27 after the index procedure and restarted on day 29.
In the post-dilation group, three definite scaffold thrombosis events occurred up to one year. The cases are described below:
CASE 1. At the time of the index procedure, the patient was treated with a 3.0×18 mm Absorb BVS in the mid LAD followed by post-dilation with a 3.25×15 mm non-compliant balloon inflated at a pressure of 12 atm. This patient subsequently presented on day 75 post procedure with acute coronary syndrome. Diagnostic angiography revealed an in-scaffold thrombosis with core lab-assessed 100% diameter stenosis. OCT at the time revealed malapposition of the scaffold. According to a platelet aggregation test, the patient was found to be resistant to clopidogrel and the dose was increased accordingly.
CASE 2. The patient was treated with a 3.0×18 mm Absorb BVS in the mid LAD coronary artery followed by post-dilation with a 3.0×9 mm non-compliant balloon inflated at a pressure of 16 atm. The patient was hospitalised on day 239 with unstable angina after being stung by a bee. Diagnostic angiography revealed a core lab-assessed 100% diameter stenosis proximal to the Absorb BVS. Aspirin and clopidogrel were ongoing at the time of the event.
CASE 3. Three initial attempts to implant a 3.0×28 mm Absorb BVS in the proximal left circumflex coronary were unsuccessful in this patient. Subsequently, two 3.0×18 mm bail-out Absorb BVS were successfully deployed across the lesion, followed by post-dilation with a 3.25×15 mm non-compliant balloon inflated at a pressure of 16 atm. The patient presented on day six post procedure with unstable angina. Diagnostic angiography revealed an in-scaffold thrombosis with a core lab-assessed 33% diameter stenosis. Aspirin and clopidogrel were ongoing at the time of the event.
Although contraindicated by the protocol, the use of a balloon catheter with a size greater than 0.5 mm above the nominal scaffold diameter occurred in 6% (30/523) of cases in the PD group. However, the use of a larger post-dilation balloon was not associated with any effect on acute gain, procedural or device success, and long-term clinical outcomes, including MACE (together with its components) and scaffold thrombosis up to one year.
Multivariable stepwise logistic regression analysis identified two independent predictors of one-year MACE events: treatment of more than one lesion (odds ratio [OR]=4.17, 95% CI=[1.41,12.5], p=0.01) and treatment of female patients (OR=2.5, 95% CI=[1.06,5.88], p=0.03). The same multivariable stepwise logistic regression analysis performed for the one-year non-Q-wave myocardial infarction (NQMI) rate identified three independent predictors: treatment of more than one lesion (OR=7.69, 95% CI=[1.92, 25.0], p=0.0035), treatment of female patients (OR=7.14, 95% CI=[2.12, 25.0], p=0.0014) and prior MI (OR=5.1, 95% CI=[1.6,16.53], p=0.0058). Therefore, post-dilation was not identified as a predictor for MACE or NQMI based on these analyses.
Discussion
The major finding of the present analysis is that, in patients with low/moderate coronary lesion complexity treated with the Absorb BVS who underwent scaffold post-dilation, a moderately increased rate of periprocedural MI was observed. However, this did not have any significant negative impact on the overall rates of cardiac death, scaffold thrombosis and ID-TLR at one year. Therefore, it is our belief that post-dilation should be performed more often following BVS implantation to optimise acute results.
Post-dilation following implantation of metallic bare metal and drug-eluting stents has become a worldwide-adopted technique in contemporary interventional cardiology practice. However, while metallic materials have high tensile strength which can potentially offer good expansion compliance of the stent without disruption risk, the introduction of the polymeric materials used in BRS has raised concerns about the expansion potential of these novel devices and the effects of high-pressure post-dilation following their deployment.
The data presented in this analysis regarding patients in whom post-dilation was not performed suggest that, when “aggressive” lesion preparation is performed prior to BRS implantation, post-dilation may not be necessary. However, certain limitations are inherent to our analyses and they need to be taken into consideration when reviewing the data and reaching subsequent conclusions.
Primarily, the analysis presented is not a randomised comparison, but rather a snapshot of real-world practice, where the decision as to whether or not to post-dilate was based solely on the investigator’s discretion rather than on clearly predefined criteria. In this scenario, it may be assumed that the decision to post-dilate was most likely based on a suboptimal angiographic result immediately following Absorb BVS deployment. Indirect evidence for this assumption can be found in the post-procedural QCA data, in which the performance of post-dilation resulted in an equivalent acute gain and residual stenosis when compared to patients without post-dilation. It is likely that both variables reflect more the different morphologic characteristics of the lesions treated in each cohort, which are not readily evaluable by QCA, rather than the performance or not of post-dilation.
Final scaffold diameter is determined by the scaffold and balloon size and by inflation pressure. However, it is also affected by the actual acute expansion and plastic deformation of the scaffold with balloon inflation and by its acute elastic recoil. Acute recoil in turn is determined by the scaffold design and the characteristics of the lesion. Onuma et al have shown a non-statistically significant but slightly higher acute recoil rate with the Absorb BVS when compared to a contemporary metallic DES with a similar design (0.19±0.18 mm for the Absorb BVS vs. 0.13±0.21 mm for the cobalt-chromium EES, p=0.4)9. However, as the same BRS was deployed in both groups of the present analysis, the lesion itself seems to be the main determinant limiting acute expansion during the Absorb BVS deployment.
Recently published by Capodanno et al, the GHOST-EU registry raised some concerns with the relatively high rate of six-month thrombosis following the unrestricted use of Absorb BVS in “real-world” practice (2.1%)10. In that trial, post-dilation was performed in 49% of the total cohort and was not correlated with better clinical outcomes in the univariate analysis. Although difficult to put our results into perspective versus the GHOST-EU registry, since the population and lesion complexity are completely different, we could only speculate whether their results would have been better if PD had been performed more often, as in the EXTEND registry (68%).
With metallic stents, Dirschinger et al demonstrated that the use of high-pressure inflation was associated with a twofold increase of peri-PCI MI rates compared to low-pressure inflation; however, this finding was not associated with any significant influence on the one-year clinical outcome11. Also of note were the significantly greater numbers of overlapping scaffolds in the post-dilation group and the longer mean length of scaffold implanted in this group, which may have influenced the occurrence of periprocedural MI. It has been well established in the literature that overlapping metallic stents is associated with a higher periprocedural rate of MI, due most likely to increased side branch occlusion along the treated segment, which can also be affected by device design and strut thickness12-15. It must be kept in mind that overlapping of an Absorb BVS results in a 300 μm layer of polymer, which is significantly thicker than that of the current metallic best-in-class DES.
Limitations
The major limitation of this study is related to its non-randomised design. However, it is very unlikely that a randomised trial of this kind will ever be performed due to the ethical implications of not performing post-dilation when a suboptimal angiographic result is observed. Additionally, the lack of routine intravascular imaging assessment (e.g., IVUS, OCT, etc.), which may better assess lesion morphology and detect subtle differences in scaffold expansion and apposition, precludes a more detailed evaluation of the cohorts. Moreover, the low-to-moderate complexity of the coronary lesions in the enrolled patients might not reflect the full spectrum of patients potentially treated with BRS in daily practice, and the differences in patient and lesion characteristics between the two groups may have impacted on the clinical results. Finally, due to the retrospective nature of this analysis, these results should be considered with caution.
Conclusions
In patients with non-complex coronary lesions undergoing BRS implantation, performing post-dilation does not negatively influence long-term clinical outcomes. Although periprocedural enzymatic myocardial infarction was observed more frequently in patients in need of post-dilation, it was not an independent predictor of periprocedural MI. Therefore, post-dilation should be performed whenever necessary to optimise acute results after BRS implantation.
Guest Editor
This paper was guest edited by Antonio Colombo, MD; Scientific Institute S. Raffaele, Milan, Italy.
| Impact on daily practice Bioresorbable scaffolds have been more frequently used in current PCI practice as an alternative to metallic stents to keep immediate vessel patency and later restore normal endothelial function. However, their use in non-selected patients has also been associated with a higher occurrence of device thrombosis within the first six months of the procedure. By performing post-dilation within the device expansion limits, operators might contribute to a better acute performance in more complex cases without significantly increasing the risks of acute complications related to device fracture. |
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

