
Abstract
Aims: This study sought to investigate the long-term clinical outcomes related to scaffold sizing based on quantitative coronary angiography.
Methods and results: A total of 1,248 patients who received Absorb bioresorbable scaffolds in the ABSORB Cohort B, ABSORB EXTEND, and ABSORB II trials were included in the analysis. The incidence of MACE (a composite of cardiac death, any myocardial infarction [MI], and ischaemia-driven target lesion revascularisation [ID-TLR]) was analysed according to the Dmax subclassification of oversized scaffold group versus non-oversized (any undersize) scaffold group. At three years, event rates were similar in both groups in MACE (9.4% vs. 9.8%, p=0.847), target vessel MI (5.2% vs. 4.8%, p=0.795), and ID-TLR (4.8% vs. 5.8%, p=0.445). Landmark analysis after one year showed that the non-oversized scaffold group had higher rates of MACE (3.2% vs. 6.9%, log-rank p=0.004), target vessel MI (0.7% vs. 2.7%, log-rank p=0.007), and ID-TLR (2.5% vs. 4.7%, log-rank p=0.041).
Conclusions: Implantation of an undersized scaffold was associated with a higher risk of MACE between one and three years, while in the previous report an oversized scaffold was associated with a higher risk of MACE up to one year. This implies different mechanisms for early and late events after scaffold implantation.
Abbreviations
BRS: bioresorbable scaffold
DES: drug-eluting stent
DOCE: device-oriented composite endpoint
ID-TLR: ischaemia-driven target lesion revascularisation
ILSD: intraluminal scaffold discontinuity
MACE: major adverse cardiac events
MI: myocardial infarction
MLD: minimal lumen diameter
NIRF: near-infrared fluorescence
OCT: optical coherence tomography
POCE: patient-oriented composite endpoint
QCA: quantitative coronary angiography
RVD: reference vessel diameter
ST: scaffold thrombosis
TLF: target lesion failure
Introduction
Bioresorbable scaffolds (BRS) were developed with the hope that they would overcome complications observed with metallic drug-eluting stents (DES) including delayed vessel healing, hypersensitivity reactions, neoatherosclerosis, and restenosis, with the risk of repeat intervention and stent thrombosis (ST)1-5. However, Absorb™ (Abbott Vascular, Santa Clara, CA, USA), the most investigated BRS, was shown to have an increased risk of target lesion failure driven by greater target vessel myocardial infarction and ischaemia-driven target lesion revascularisation in comparison with the contemporary XIENCE DES (Abbott Vascular)6. Even between one and three years, when the device is expected to have been well incorporated into the vessel wall, rates of target lesion failure and device thrombosis were higher with BRS than with DES.
Shortly after scaffold implantation, coronary arteries show an alternans of extremely low and high endothelial shear stress values and localised areas of high blood viscosity. These initial local haemodynamic disturbances may trigger fibrin deposition and thrombosis7. On the other hand, at a later phase, these heterogeneities in shear stress would be normalised and other factors such as scaffold discontinuity and neoatherosclerosis would emerge as possible causes of adverse events8.
Previously, we have reported that scaffold oversizing is associated with major adverse cardiac events at one year in a pooled population of ABSORB Cohort B, ABSORB EXTEND, and ABSORB II9. However, as suggested above, different aetiologies may contribute to the occurrence of early (~one year) and late adverse events. Therefore, we aimed to investigate the relationship between events and scaffold sizing based on quantitative coronary angiography (QCA) with longer-term outcome (~three years) and to explore possible causes of late scaffold failure.
Methods
STUDY DESIGN AND POPULATION
We analysed the results of Absorb scaffold implantation in 1,248 patients enrolled between 2009 and 2013 in the ABSORB Cohort B study10, ABSORB EXTEND study11, and ABSORB II12 randomised controlled trial. The design of each study is described elsewhere13-15. In the ABSORB Cohort B, a 3.0/18 mm Absorb scaffold was available. In the ABSORB EXTEND and ABSORB II studies, patients were treated as follows14,15: 1) a 3.5 mm Absorb scaffold was used when both the proximal and distal Dmax were within an upper limit of 3.8 mm and a lower limit of 3.0 mm; 2) a 3.0 mm Absorb scaffold was used when both the proximal and distal maximal lumen diameters were within an upper limit of 3.3 mm and a lower limit of 2.5 mm; 3) a 2.5 mm Absorb scaffold was used when both the proximal and the distal Dmax were within an upper limit of 3.0 mm and a lower limit of 2.25 mm; and 4) scaffold overlap was allowed. All of these trials were sponsored and funded by Abbott Vascular. The research ethics committee of each participating institution approved the protocol, and all enrolled patients provided written informed consent before inclusion.
STUDY DEVICE
The details of the study device (Absorb) have been described in detail previously15. In brief, the Absorb scaffold comprises a poly-L-lactide backbone coated with an amorphous drug-eluting coating matrix composed of poly-D,L-lactide polymer containing everolimus. According to preclinical studies, the time for complete bioresorption of the polymer backbone is two to three years16.
QCA ANALYSIS
QCA guidance of Absorb implantation relies on the angiographic diameter function curve of the pre-treatment vessel segment that contains three unambiguous luminal dimensions, namely the minimal lumen diameter (MLD), and the maximal luminal diameter (Dmax) in the proximal segment (proximal Dmax) and in the distal segment (distal Dmax) with respect to the MLD9. QCA analyses were undertaken by the sites before Absorb implantation, and post-procedurally by an independent core laboratory (Cardialysis BV, Rotterdam, the Netherlands) using a Cardiovascular Angiography Analysis System (Pie Medical Imaging, Maastricht, the Netherlands).
DEFINITIONS AND ENDPOINTS
The patient population in the present study was stratified by the difference between the angiographic maximal diameter and the nominal diameter of the implanted scaffold. The selection of device size was considered “oversized” (oversized scaffold group) when the patient received one or more devices in vessels in which both the proximal and the distal Dmax were smaller than the nominal size of the device. Patients who received Absorb scaffolds in vessels with either a proximal or a distal Dmax or both Dmax larger than the nominal size of the device constituted the “non-oversized scaffold group” (in other words, “(any) undersized scaffold group”). When a patient received two or three overlapping Absorb scaffolds in a long lesion, the nominal size of the proximally implanted device was compared with the proximal Dmax, whereas the nominal size of the distally implanted device was compared with the distal Dmax.
In the present analysis, we followed the same definitions of clinical outcomes as in the previous report9. In brief, the primary outcome of interest was major adverse cardiac events (MACE), defined as a composite of cardiac death, any myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (ID-TLR). Device-oriented composite endpoint (DOCE) was defined as a composite of cardiac death, target vessel MI, and ID-TLR. Patient-oriented composite endpoint (POCE) was defined as a composite of all death, all MI, and all revascularisation. MI classification and criteria for diagnosis were defined according to the per-protocol definition, which was consistently applied in all trials included in the present analysis12. Definite or probable scaffold thrombosis (ST) was adjudicated according to the Academic Research Consortium definitions17. All clinical outcomes were adjudicated by an independent clinical events committee.
STATISTICAL ANALYSIS
All analyses were conducted using the intention-to-treat population. The present analyses were based on individual patient-level data. Categorical variables were compared by Fisher’s exact test. Continuous variables were presented as mean ±SD and were compared by t-test. Time-to-event variables were presented as Kaplan-Meier curves and the Shoenfeld residuals test was performed. If the proportional hazard could be assumed, a log-rank test was carried out subsequently. Landmark analyses were performed at one year after the index procedure. To determine the independent predictors of MACE between one and three years, firstly univariate logistic regression models were constructed using the following variables: age, male sex, current smoking, hypertension requiring treatment, dyslipidaemia requiring treatment, any diabetes, unstable angina, prior myocardial infarction, preprocedural diameter stenosis, preprocedural MLD, preprocedural reference vessel diameter (RVD), lesion length, smallest (Dmax – device diameter), smallest angulation >45°, calcified lesions, preprocedural visible thrombus, bifurcation lesions, type B2/C lesions, left anterior descending artery, nominal scaffold size/post-procedural MLD, treatment with overlapping scaffolds, target vessel treatment with 2.5 mm device, and scaffold implantation in a vessel with both proximal and distal Dmax smaller than the nominal device size. Secondly, significant variables (p<0.15) in the univariate analysis were forcedly entered into a multivariable logistic regression model to predict for MACE between one and three years. A two-sided p-value <0.05 was considered significant for all tests. Statistical tests were performed with SPSS Statistics 24.0.0.2 (IBM Corp., Armonk, NY, USA) and R, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of a total population of 1,248 patients, preprocedural Dmax was assessed by the core laboratory in 1,232 (98.7%) patients. The nominal size of the implanted Absorb scaffold was larger than both proximal and distal Dmax in 649 patients (oversized scaffold group 52.7%).
Clinical and angiographic characteristics between the oversized scaffold group and the non-oversized scaffold group are given in the previous report9 and tabulated in Supplementary Table 1. The two groups did not differ significantly with regard to main baseline clinical characteristics, whereas preprocedural MLD, reference vessel diameter, and both proximal and distal Dmax were significantly smaller in the oversized scaffold group than in the non-oversized scaffold group. The study flow chart is shown in Figure 1.
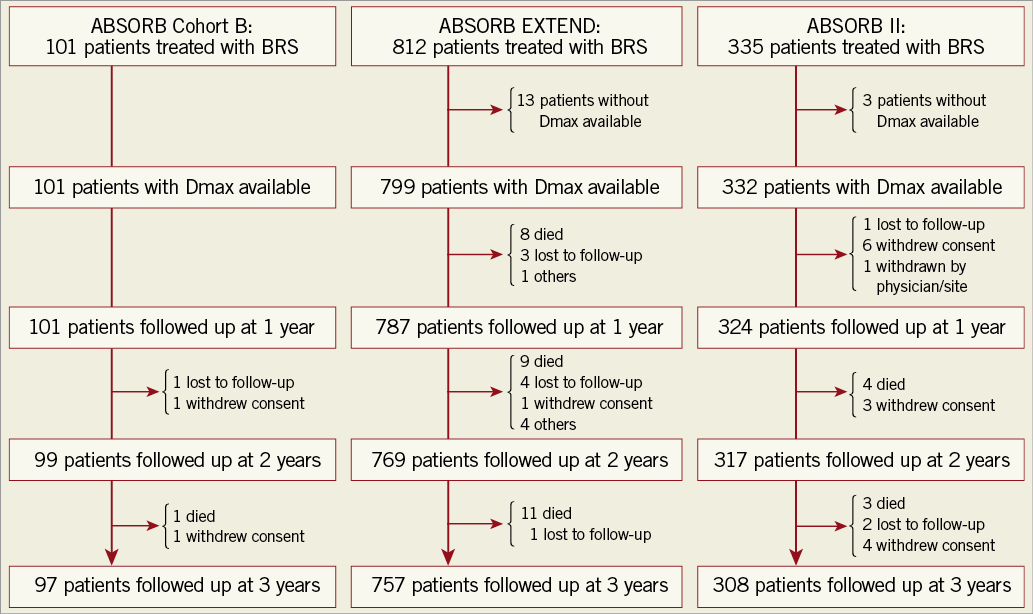
Figure 1. Study flow chart. BRS: bioresorbable scaffold
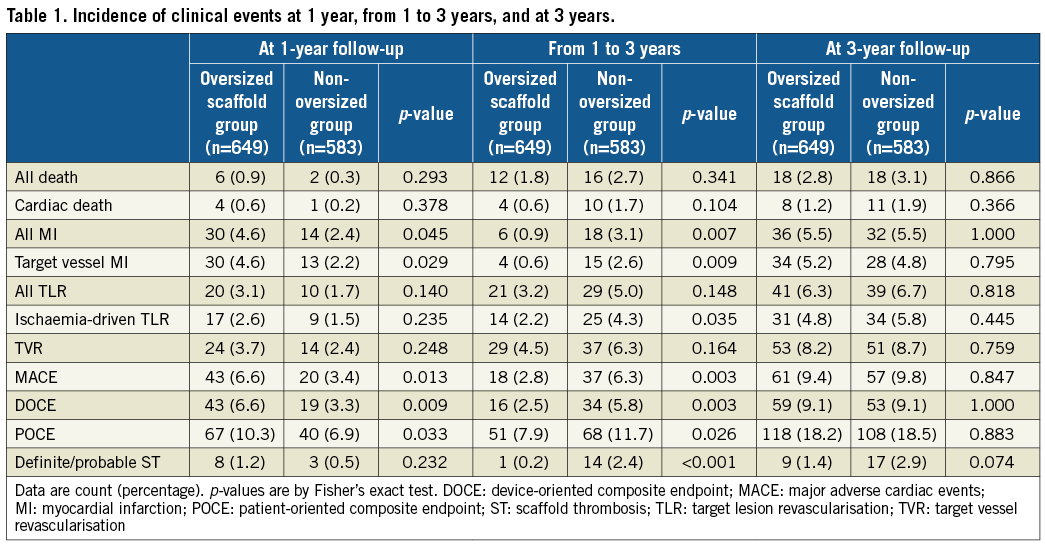
Comparisons of event rates between the oversized scaffold group and the non-oversized group at one year, between one and three years, and at three years are shown in Table 1. At one year, the oversized scaffold group was associated with a higher risk of MACE and POCE mainly driven by all MI, as well as DOCE mainly driven by target vessel MI. However, at three years, event rates were similar in MACE (9.4% vs. 9.8%, p=0.847), all MI (5.5% vs. 5.5%, p=1.000), target vessel MI (5.2% vs. 4.8%, p=0.795), and ID-TLR (4.8% vs. 5.8%, p=0.445).
Kaplan-Meier curves are shown in Figure 2. Landmark analyses from one to three years show that the non-oversized group had a significantly higher rate of MACE than the oversized group, driven by significant differences in target vessel MI and ID-TLR, resulting in the similar rate of clinical events at three years. A log-rank test at three years was not performed due to violation of proportional hazards assumption (Supplementary Table 2).
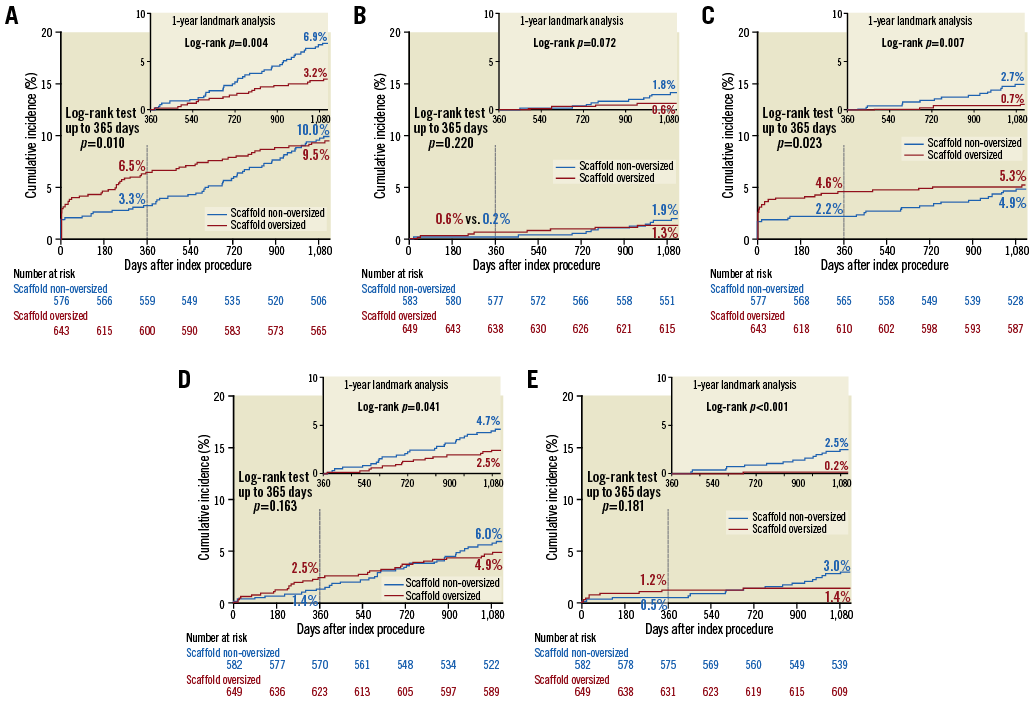
Figure 2. Time-to-event curves of MACE and its components. Three-year and one-year landmark analysis (upper right panel) of time-to-event curves of MACE (A) and its components (B: cardiac death; C: target vessel MI; D: ID-TLR) and of definite/probable ST (E) according to study group. Log-rank test at three years was not performed due to violation of proportional hazards assumption (Supplementary Table 2). ID-TLR: ischaemia-driven target lesion revascularisation; MACE: major adverse cardiac events; MI: myocardial infarction; ST: scaffold thrombosis
The graphical presentation as a function of (distal Dmax minus nominal scaffold size) and (proximal Dmax minus nominal scaffold size) clearly shows that those patients with MACE clustering in the oversized group in the lower left quadrant at one year have moved in the upper right direction between one and three years (Figure 3A). Notably, definite/probable scaffold thrombosis cases showed more clearly that lower left clustering of event cases at one year had moved in the upper right direction (Figure 3B).
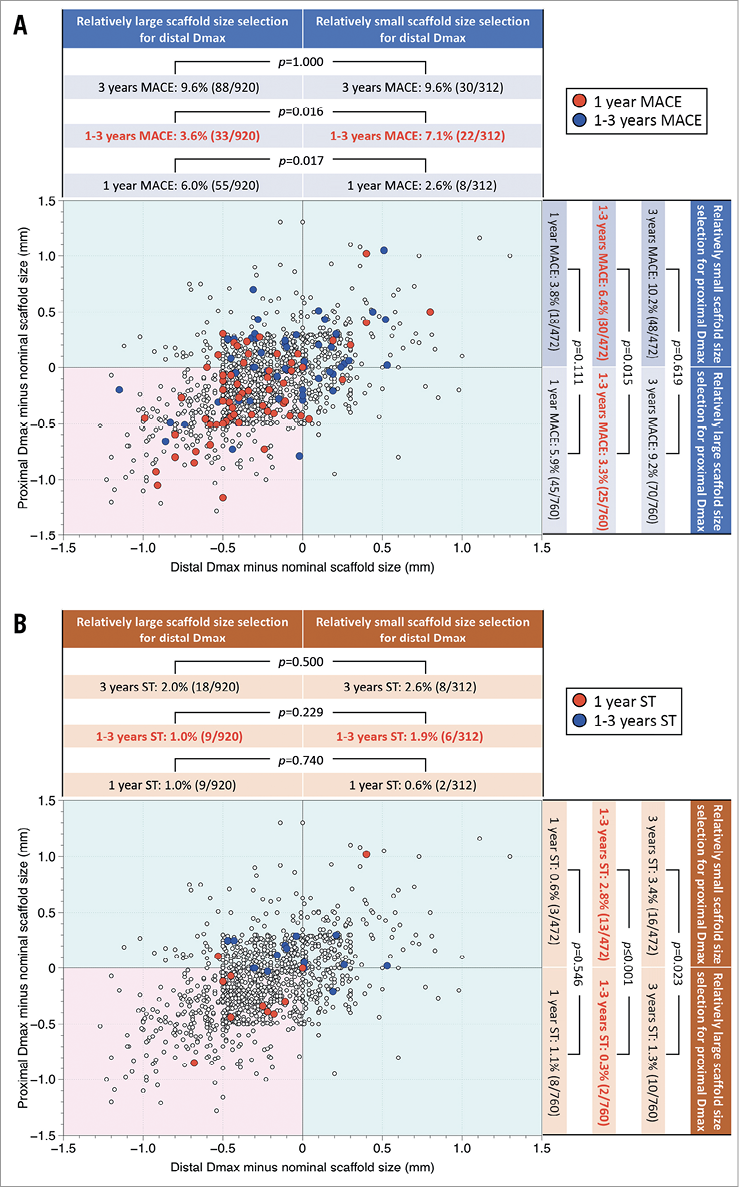
Figure 3. MACE and ST distribution as a function of difference between Dmax and nominal scaffold diameter. A) Distribution of proximal and distal Dmax measurements minus nominal scaffold size in patients with or without major adverse cardiac events (MACE) is shown. The red and blue filled circles represent the patients who experienced MACE at one year and during one to three years, respectively. The graphical presentation clearly shows that those patients with MACE clustering in the oversized group in the lower left quadrant (pink square) at one year had moved in the upper right direction between one and three years. B) Distribution of proximal and distal Dmax measurements minus nominal scaffold size in patients with or without definite/probable scaffold thrombosis (ST) is shown. The red and blue filled circles represent the patients who experienced definite/probable ST at one year and during one to three years, respectively. The graphical presentation clearly shows that those patients with ST clustering in the oversized group in the lower left quadrant (pink square) at one year had moved in the upper right direction between one and three years.
By multivariable analysis, type B2 or C lesions (odds ratio [OR]: 0.49, 95% confidence interval [CI]: 0.27 to 0.88, p=0.018) and the oversized group (OR 0.49, 95% CI: 0.24 to 0.97, p=0.041) were negatively associated with one- to three-year MACE (Table 2 [results of univariate analyses in Supplementary Table 3]). In other words, the presence of any undersizing was a (positive) independent predictor of one- to three-year MACE.
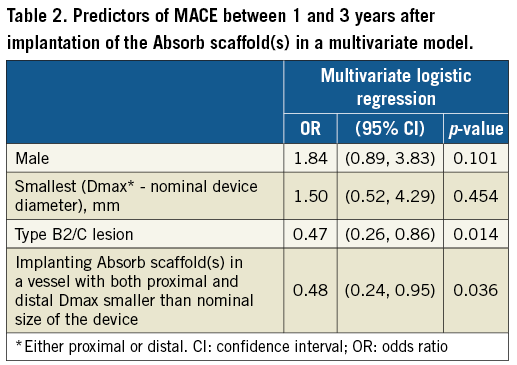
Discussion
Although in the multivariate analysis scaffold oversizing was independently associated with MACE up to one year in the previous report9, scaffold oversizing turned out to be an independent protective factor between one and three years in the current study, resulting in the similar MACE rate between the oversized and non-oversized groups at three years. Namely, scaffold non-oversizing (any undersizing) was significantly associated with late adverse clinical outcome.
Recently, in the MICAT single-centre registry (n=657), Gori et al reported that scaffold oversizing was associated with a higher incidence of early ST, while undersizing was associated with ST at a later phase18. The results in the present study are in line with their report; however, it is of note that the Dmax analysis performed by the core lab led to the same conclusion in the present study.
POSSIBLE CAUSES OF EARLY SCAFFOLD FAILURE
Compared with contemporary metallic DES, the first-generation BRS has substantially thicker and wider struts, which induces greater shear stress and platelet activation, more frequently obstructs side branches19, and prolongs the time required for complete endothelialisation. These factors may be exacerbated when a relatively large scaffold is implanted in a relatively small vessel, increasing the density of scaffold struts and thereby inducing early events such as periprocedural MI (Figure 4). At a later phase, struts would be covered by neointima, smoothening the lumen-strut surface, and the impact of those factors may become less.
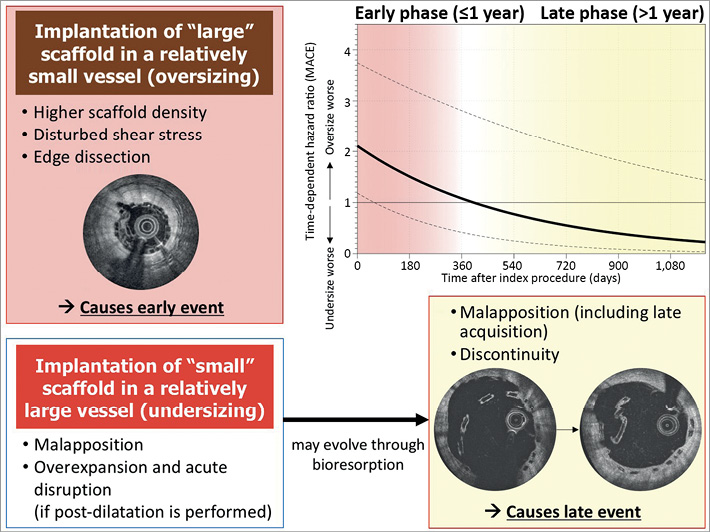
Figure 4. Impact of scaffold sizing over time and possible causes of early and late scaffold failure. The upper right panel shows time-dependent hazard ratio comparing oversizing vs. any undersizing on MACE. The regression line was based on Cox regression analysis including time after index procedure as an interaction term. The dotted lines indicate 95% confidence interval of hazard ratio. MACE: major adverse cardiac events
POSSIBLE CAUSES OF LATE SCAFFOLD FAILURE
Possible consequences after implanting a relatively small scaffold in a relatively large vessel are 1) malapposition and 2) acute disruption due to overexpansion if post-dilatation with a large balloon is performed.
Although malapposition was frequently observed in scaffold thrombosis cases20, an OCT substudy of ABSORB Japan showed that the %malapposed strut and incomplete strut apposition area observed post-procedure in BRS decreased in two years, from 4.8% to 0.1% (p=0.001) and from 0.10 mm2 to 0.01 mm2 (p<0.001), respectively21, suggesting little impact of the malapposition observed post-procedure on late outcome. Acute disruption was not a frequent cause of early or late scaffold thrombosis20; however, this entity may be overlooked and later diagnosed as late discontinuity, especially when acute disruption was too discrete to be diagnosed even on high-speed OCT.
Translocation of scaffold struts into the lumen during the bioresorption process between one and three years (intraluminal scaffold discontinuity [ILSD]) has emerged as a unique cause of very late device thrombosis that has not been seen with metallic DES. Even if the struts are completely apposed at baseline, expansive remodelling, more frequently observed in BRS22, could promote late acquired malapposition. In the presence of relevant areas of malapposed or uncovered scaffold struts, late scaffold discontinuity related to the resorption process may cause dislocation of strut remnants into the lumen with subsequent activation of the coagulation cascade, triggering very late scaffold thrombosis23. Indeed, if we apply a time-dependent Cox model to our study population (Figure 4), the hazard ratio of MACE (oversizing vs. [any] undersizing) went below one at one year, exactly the time when the scaffold completely loses mechanical support24. Recently, a report of the INVEST registry demonstrated that discontinuity, malapposed struts, and uncovered struts are significantly more frequent in the thrombosed than in the non-thrombosed scaffold regions8. However, the mechanism leading to scaffold thrombosis is still unclear. In the population of the INVEST registry, strut discontinuity was preceded by malapposition at the time of device implantation in one case, while two patients with serial OCT exhibited scaffold discontinuity preceded by a state of full apposition and strut coverage. Struts with discontinuity embedded in neointima have been reported frequently (i.e., 22% of lesions at two years and 42% at three years) and were not associated with clinical sequelae in the ABSORB Cohort B25. Discontinuity alone may be benign but would trigger late events if it occurs together with malapposition or uncoverage.
Other factors which may contribute to late events are inflammation and neoatherosclerosis. In a porcine study by Otsuka et al26, inflammation surrounding struts was greater in BRS at six to 36 months than in metallic DES. Inflammation in neointima could accelerate neoatherosclerotic change through impaired endothelial function27. An OCT-NIRF study in a porcine coronary artery showed that BRS disruption was strongly associated with arterial inflammation, leading to excessive neointimal proliferation and restenosis, suggesting an interaction among late discontinuity, inflammation, and adverse outcome.
The reason why B2/C lesions were protective for later events in the present study is unclear. However, a possible explanation is that B2/C lesions prompted the operator to perform more aggressive lesion preparation such as rotational atherectomy or use of a cutting balloon. While these techniques could sometimes be related to periprocedural complications, they could modify the impact of lesion characteristics at a later phase.
PROPOSED SOLUTION TO IMPROVE OUTCOME IN LESIONS TREATED WITH BRS
The impact of operator technique was investigated in a report aggregating data from ABSORB trials (n=2,973)28. Vessel sizing, defined as selection of lesions with a reference vessel diameter ≥2.25 mm and ≤3.75 mm, was shown to be a significant predictor of target lesion failure up to one year, but not between one and three years. This result suggests the importance of lesion selection especially for the early phase (up to one year); however, it should be noted that the relative relationship between vessel size and scaffold diameter was not taken into account in the report28. Even with meticulous analysis of relative scaffold sizing using Dmax, we could not come to an appropriate recommendation of sizing as there is no clear area of (mis)matching without MACE (Figure 3A). For future studies, vessel sizing by OCT after predilatation is recommended to reflect the true luminal dimension before scaffold implantation. Optimal post-dilatation (with a non-compliant balloon at ≥18 atm, larger than the nominal scaffold diameter, but not by more than 0.5 mm) seems reasonable since it reduced the TLF rate between one and three years. In contrast, the significance of an aggressive predilatation is unclear since it increased the TLF rate up to one year, although it was an independent predictor of freedom from scaffold thrombosis between one and three years28.
Limitations
The information on predilatation and post-dilatation was not available in all cases and could not be incorporated in the multivariate analysis. The omission of events occurring earlier than the landmark is among the recognised limitations of a landmark analysis29. Nevertheless, the time-dependent Cox regression model indicated a reverse of the hazard ratio for MACE at one year between an oversized scaffold and a scaffold with any undersizing. It remains to be determined whether modifications in scaffold design can mitigate the risk of device failure.
Conclusions
Implantation of an undersized Absorb scaffold in a relatively large vessel was associated with a higher risk of MACE between one and three years, while an oversized Absorb scaffold in a relatively small vessel was associated with a higher risk of MACE at one year. Various cascades leading to an early or late adverse event were considered, but mechanistic aetiologies should be elucidated further in imaging studies.
| Impact on daily practice The present study demonstrated that implantation of an undersized Absorb scaffold is associated with a higher risk of MACE between one and three years, while an oversized Absorb scaffold is associated with a higher risk of MACE at one year. Various cascades leading to an early or late adverse event were considered, but mechanistic aetiologies should be elucidated further in imaging studies. |
Guest Editor
This paper was guest edited by Antonio Colombo, MD, FACC, FESC, FSCAI; Department of Interventional Cardiology, EMO GVM Centro Cuore Columbus, Milan, Italy, and by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard and University Paris VII, Paris, France.
Funding
This study was funded by Abbott Vascular.
Conflict of interest statement
Y. Onuma has served as a member of the advisory board for Abbott Vascular and has received speaker’s honoraria from Terumo. J. Piek has served as a member of the advisory board for Abbott Vascular. B. Chevalier has served as a consultant for Abbott Vascular. P. Serruys has served as a member of the advisory board for Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor, A. Colombo, has no conflicts of interest to declare. The Guest Editor, A. Vahanian, is a consultant for Edwards Lifesciences.
Supplementary data
Supplementary Table 1. Clinical, and preprocedural and post-procedural angiographic characteristics.
Supplementary Table 2. Schoenfeld residuals test to evaluate proportional hazards assumption.
Supplementary Table 3. Univariate analysis of MACE between 1 and 3 years after implantation of the Absorb scaffold(s).
To read the full content of this article, please download the PDF.

