Abstract
Aims: The aim of the study was to evaluate the impact of bioresorbable vascular scaffold (BRS) implantation technique on post-procedural quantitative coronary angiography (QCA) parameters in ST-elevation myocardial infarction (STEMI).
Methods and results: We assessed 442 STEMI patients who underwent BRS implantation in the BVS STEMI STRATEGY-IT study. Optimal BRS implantation was assessed using the PSP score, developed and validated in the GHOST-EU registry. We analysed post-implantation QCA parameters, including minimum lumen diameter (MLD) and maximum footprint, in patients with and without optimal BRS implantation, coded as maximum PSP score. Patients with optimal BRS implantation had higher post-procedural MLD and lower maximum footprint than those without. Multivariate analysis demonstrated that optimal BRS implantation was an independent predictor of high post-procedural MLD, defined as ≥2.4 mm for 2.5 or 3.0 mm BRS and ≥2.8 mm for 3.5 mm BRS. Thrombectomy before optimal BRS implantation showed a trend towards higher post-procedural MLD and lower maximum footprint. There was no relationship between optimal BRS implantation and device-oriented composite events at one year.
Conclusions: Optimal BRS implantation, as assessed by PSP score, was associated with better post-procedural QCA parameters in STEMI. Thrombectomy before optimal BRS implantation might improve angiographic results in STEMI. Long-term follow-up is needed to analyse the relationship between QCA parameters and clinical outcomes after BRS implantation in STEMI patients.
Abbreviations
| BRS | bioresorbable vascular scaffold |
| MLD | minimum lumen diameter |
| PCI | percutaneous coronary intervention |
| QCA | quantitative coronary angiography |
| STEMI | ST-elevation myocardial infarction |
Introduction
Bioresorbable vascular scaffolds (BRS) were developed to overcome the limitations of metallic stents, including delayed arterial healing and chronic inflammation1,2,3. Nevertheless, as compared to metallic stents, the Absorb™ BRS (Abbott Vascular, Santa Clara, CA, USA) has shown an increased risk of thrombosis4 with higher acute recoil and lower post-procedural minimum lumen diameter (MLD) due to low radial strength5. Post-procedural MLD, scaled residual stenosis, and scaffold maximum footprint were shown to be surrogate markers for predicting scaffold thrombosis (ScT)6. Optimised BRS implantation may reduce the incidence of ScT6.
The predilation, scaffold sizing, and post-dilation score (PSP score) has been proposed to evaluate the quality of BRS implantation in an all-comer population and to predict device-oriented events7. No data are available on its value in STEMI patients with a high thrombotic burden8, and specifically on whether optimal BRS implantation may affect post-procedural quantitative coronary angiography (QCA) parameters.
Therefore, we sought to analyse the impact of BRS implantation technique, as evaluated by the PSP score, on post-procedural QCA parameters in STEMI patients.
Methods
STUDY POPULATION
This retrospective analysis used data obtained from the BVS STEMI STRATEGY-IT study (ClinicalTrials.gov Identifier: NCT02601781). The inclusion and exclusion criteria, the rationale and results of this study have been reported previously9. Briefly, this is an investigator-initiated, prospective, non-randomised, single-arm multicentre study on consecutive STEMI patients who underwent primary PCI with BRS implantation at 22 hospitals in Italy. The study protocol was developed in accordance with the Declaration of Helsinki and was approved by the ethics committee of each participating hospital. All patients provided written informed consent prior to study participation.
PROCEDURAL DETAILS AND DEFINITION OF PSP SCORE
BRS implantation procedures were performed based on a pre-specified BRS implantation strategy (Supplementary Figure 1) and scored using the PSP scoring system (Supplementary Table 1),7,9. The PSP score is a simple score model, developed and validated in the GHOST-EU registry, whose objective is to assess the relationship between the quality of BRS implantation technique and adverse clinical events.
ASSESSMENT OF QUANTITATIVE CORONARY ANGIOGRAPHY
QCA analysis was performed offline at the independent core laboratory of the Hospital Clinic of Barcelona, using the QAngio XA analysis system (Medis medical imaging systems bv, Leiden, the Netherlands). For each lesion, the scaffolded segment and the peri-scaffold segments (defined by a length of 5 mm proximal and distal to the scaffold edge) were analysed, as previously reported10. The following QCA parameters were measured: post-procedural MLD, acute gain, acute absolute recoil and maximum footprint10. The primary objective of this study was to evaluate the relationship between optimal BRS implantation and these QCA parameters.
CLINICAL OUTCOMES
The following clinical outcomes were assessed: device-oriented composite endpoint (DoCE) and scaffold thrombosis (ScT) at one year. DoCE was a composite of cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularisation.
STATISTICAL ANALYSIS
Continuous variables were assessed for normal distribution using the Shapiro-Wilk test and are presented as mean±standard deviation or median and interquartile range (IQR), as appropriate. Dichotomous variables are described as numbers and percentages. All patients were stratified into two groups according to optimal BRS implantation, coded as maximum or highest PSP score value. Differences between the two groups were compared using the chi-square test for categorical variables and Student’s t-tests or Wilcoxon rank-sum tests for continuous variables.
High post-procedural MLD was defined as follows: 1) post-MLD ≥2.4 mm for 2.5 or 3.0 mm BRS, and 2) post-MLD ≥2.8 mm for 3.5 mm BRS, based on the definition in a recent study6. Based on the same study, low maximum footprint was defined as <36%6.
Multivariate logistic regression analysis was performed to identify independent predictors of high post-procedural MLD and low maximum footprint. Variables that showed a p-value <0.1 in univariate analysis were entered into the multivariate model. Finally, we assessed the impact of optimal BRS implantation on post-procedural QCA parameters by adjusting for male sex, diabetes mellitus, bifurcation, right coronary artery (RCA) lesion, preprocedural reference vessel diameter, direct implantation, and thrombectomy. To assess the weight of each BRS implantation technique step on QCA parameters, we compared post-procedural QCA parameters between each implantation step, categorised as a binary variable (accomplished/not accomplished), using the Wilcoxon rank-sum test.
Statistical analysis was performed using the Statistical Package for Social Sciences, Version 21 (IBM Corp., Armonk, NY, USA) software. A two-tailed p-value <0.05 was considered as statistically significant.
Results
BASELINE CLINICAL AND PROCEDURAL CHARACTERISTICS
Out of 505 patients included in the BVS STEMI STRATEGY-IT study, 63 patients (12.5%), in whom a low-quality angiogram did not allow QCA analysis, were excluded. The remaining 442 patients (87.5%) were included in this study. There were no differences in baseline characteristics between included and excluded patients (Supplementary Table 2).
Overall, patients with optimal BRS implantation were 12.9% (n=57) in category PSP-1, 13.8% (n=61) in category PSP-2, and 8.4% (n=37) in category PSP-3. Patients’ clinical and procedural characteristics are shown in Table 1 and Table 2. Patients with optimal BRS implantation had a significantly higher rate of RCA culprit lesions and of 3.0 mm scaffolds implanted than those without; this difference was consistent within the PSP-1, PSP-2, and PSP-3 groups (Supplementary Table 3-Supplementary Table 5).
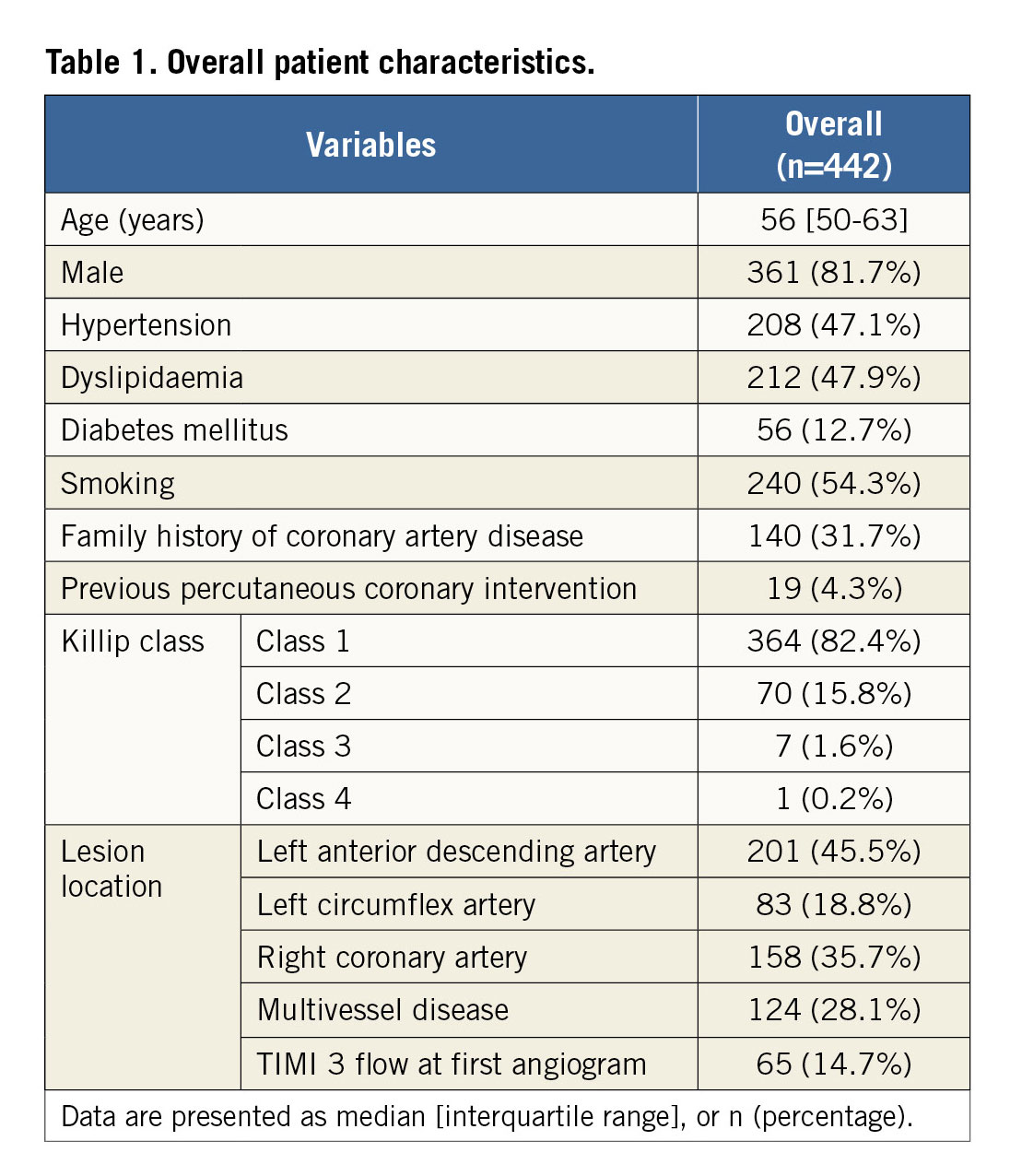
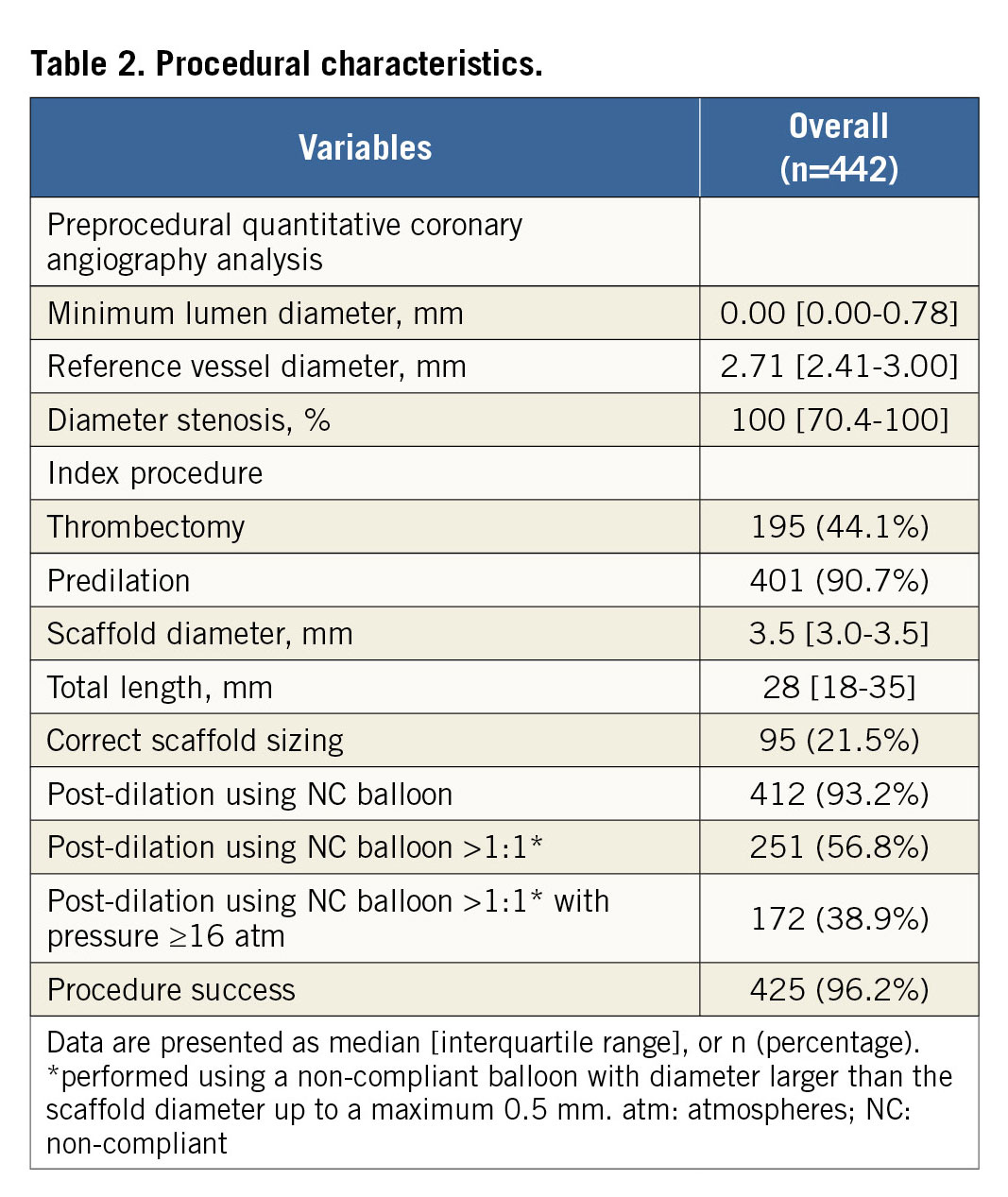
RELATIONSHIP BETWEEN POST-PROCEDURAL QCA PARAMETERS AND OPTIMAL PSP TECHNIQUE
Post-procedural QCA parameters are shown in Figure 1. Patients with optimal BRS implantation had significantly higher post-procedural MLD than those without (PSP-1: 2.75 mm [IQR: 2.41-2.91] vs. 2.52 mm [IQR: 2.25-2.79], p=0.002; PSP-2: 2.75 mm [IQR: 2.44-2.91] vs. 2.52 mm [IQR: 2.24-2.78], p<0.001; PSP-3: 2.73 mm [IQR: 2.34-2.91] vs. 2.54 mm [IQR: 2.27-2.80], p=0.084) (Figure 1A, Figure 1B). The optimal PSP technique was significantly associated with lower maximum footprint compared to non-optimal PSP technique (PSP-1: 19.9% [IQR: 12.8-32.8] vs. 31.9% [IQR: 20.6-44.2], p<0.001; PSP-2: 19.8% [IQR: 12.8-32.6] vs. 32.0% [IQR: 21.4-44.4], p<0.001; PSP-3: 21.8% [IQR: 13.4-36.1] vs. 30.8% [IQR: 19.9-43.3], p=0.006) (Figure 1C). There were no differences in acute gain (Figure 1D) and acute absolute recoil (Figure 1E) between optimal and non-optimal BRS implantation.
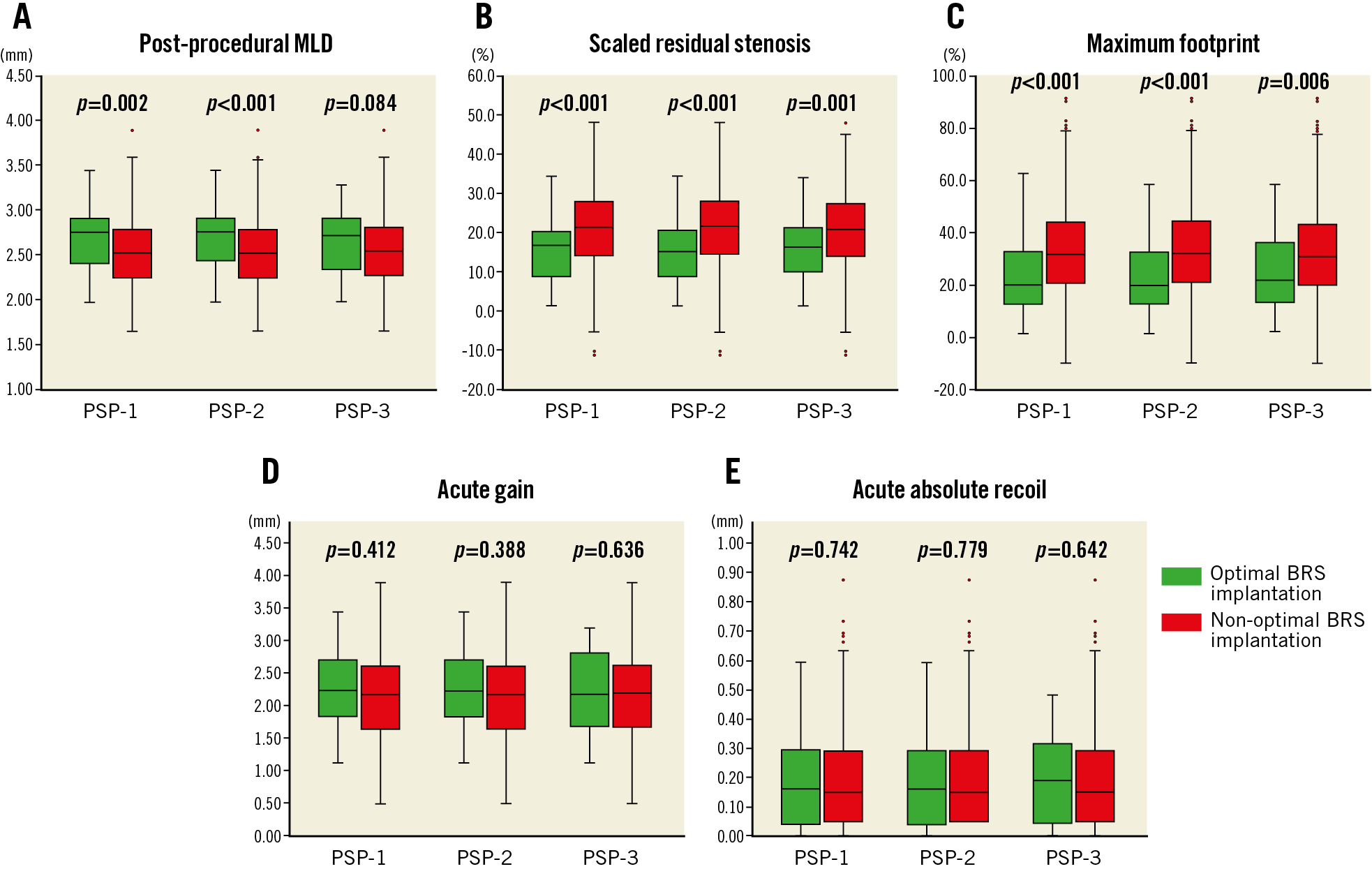
Figure 1. Optimal BRS implantation and post-procedural quantitative coronary angiography parameters. A) Optimal BRS implantation was significantly associated with higher post-procedural MLD than non-optimal BRS implantation (PSP-1: 2.75 mm vs. 2.52 mm, p=0.002; PSP-2: 2.75 mm vs. 2.52 mm, p<0.001; PSP-3: 2.73 mm vs. 2.54 mm, p=0.084). B) Optimal BRS implantation was associated with lower scaled residual stenosis compared to non-optimal BRS implantation (PSP-1: 15.3% vs. 21.4%, p<0.001), (PSP-2: 15.0% vs. 21.6%, p<0.001), (PSP-3: 16.3% vs. 20.9%, p=0.001). C) Optimal BRS implantation was also significantly associated with lower maximum footprint compared to non-optimal BRS implantation (PSP-1: 19.9% vs. 31.9%, p<0.001), (PSP-2: 19.8% vs. 32.0%, p<0.001), (PSP-3: 21.8% vs. 30.8%, p=0.006). D) & E) Acute gain and acute absolute recoil were not associated with PSP scores. MLD: minimum lumen diameter
The multivariate analysis demonstrated that predictors of high post-procedural MLD were optimal BRS implantation, thrombectomy, RCA lesion, and preprocedural reference vessel diameter (Table 3). On the other hand, optimal BRS implantation was not an independent predictor of low maximum footprint (Supplementary Table 6). Because thrombectomy had an impact on post-procedural QCA parameters in multivariate analysis, we assessed whether the combination of thrombectomy with optimal BRS implantation may affect post-procedural QCA parameters or not. Thrombectomy plus optimal BRS implantation was performed in 6.3% of patients (n=28) in the PSP-1 group, 7.0% of patients (n=31) in the PSP-2 group, and 4.3% of patients (n=19) in the PSP-3 group. The combination of thrombectomy with optimal BRS implantation exhibited a trend towards higher post-MLD and lower maximum footprint in the STEMI population (Figure 2).
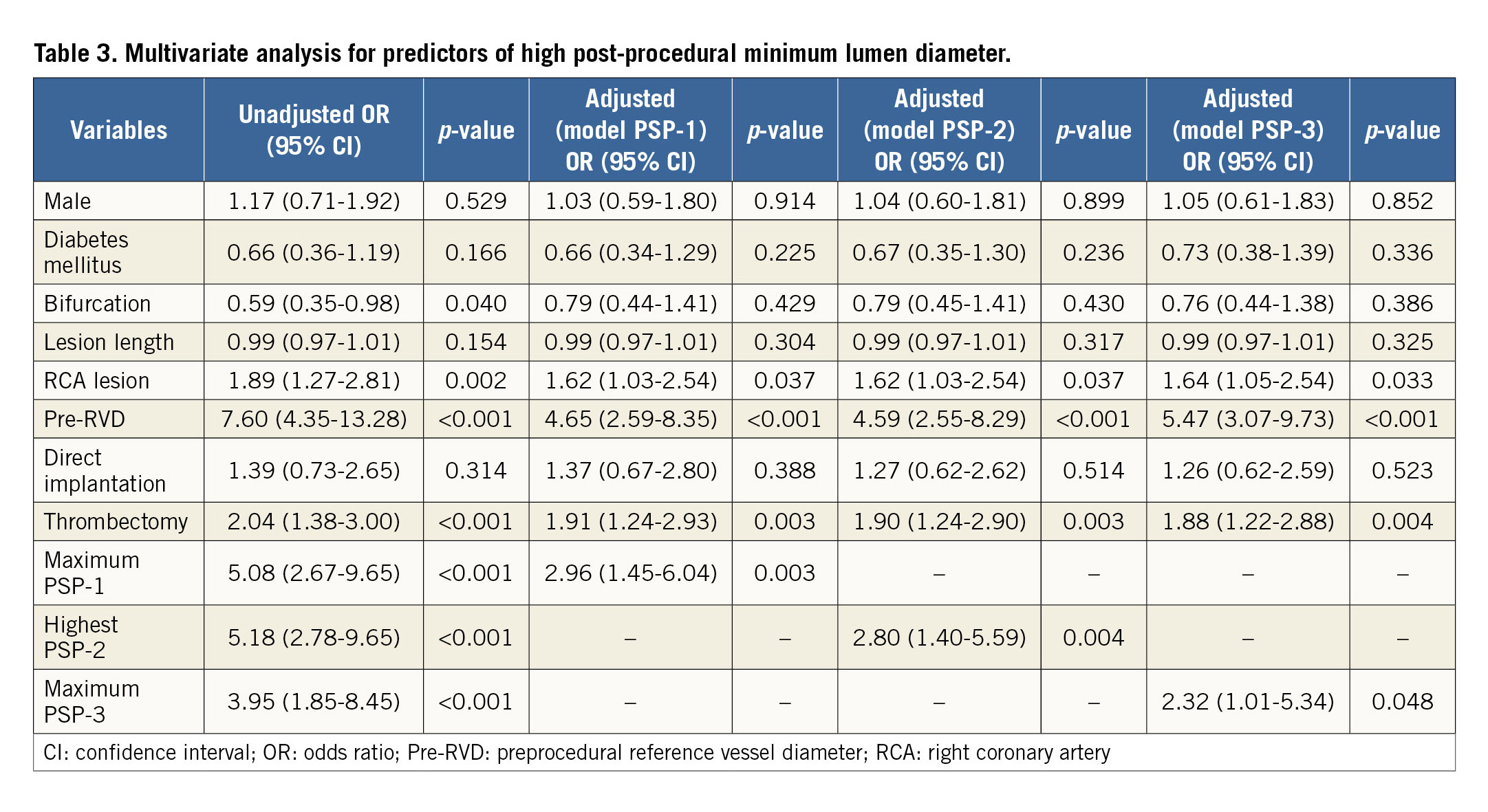
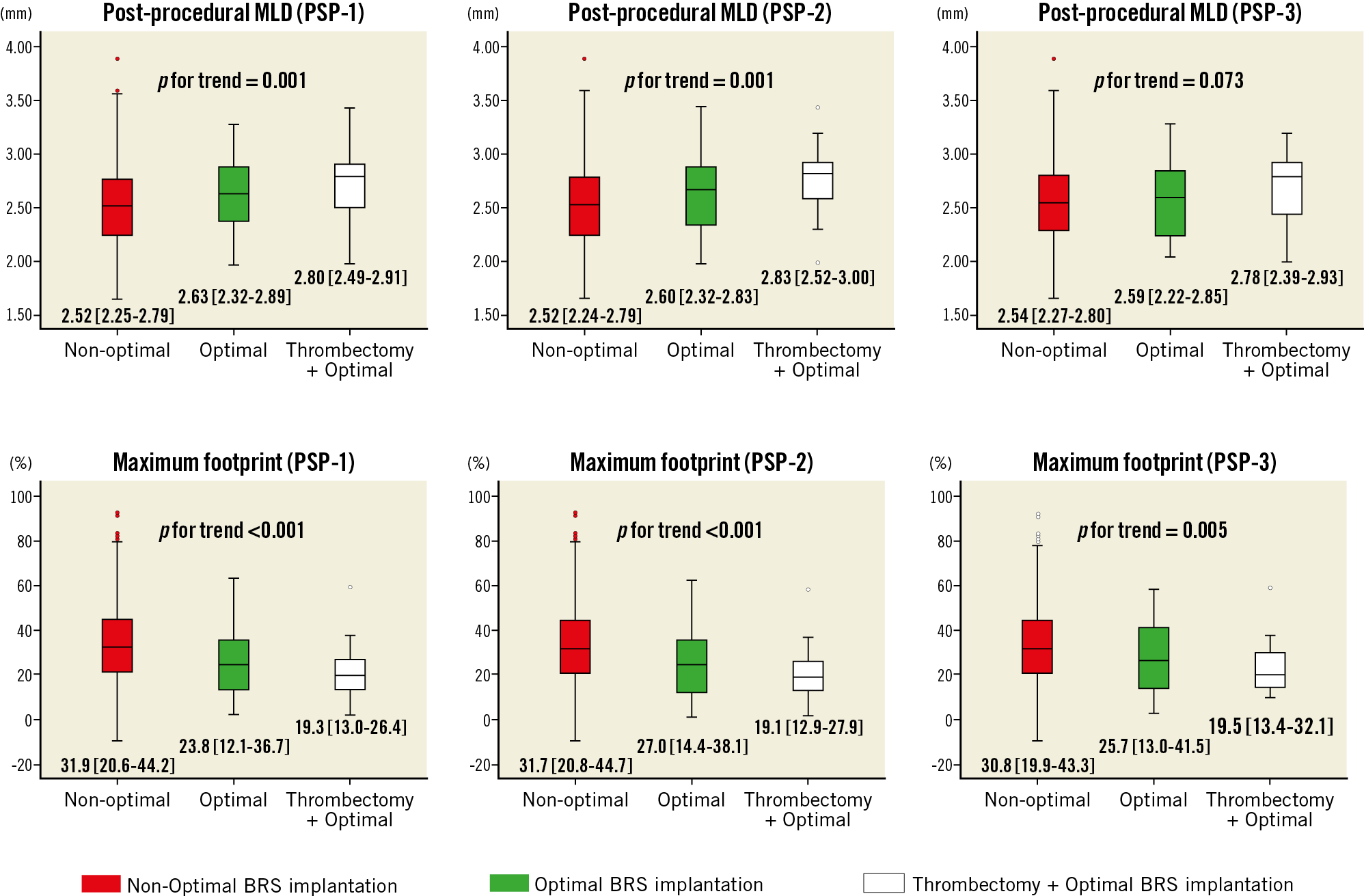
Figure 2. Combination of thrombectomy with optimal BRS implantation and post-procedural quantitative coronary angiography parameters. A combination of thrombectomy and optimal BRS implantation was associated with higher post-procedural minimum lumen diameter (upper row) and lower maximum footprint (lower row), irrespective of PSP model. Non-optimal: non-optimal BRS implantation; Optimal: optimal BRS implantation
IMPLANTATION TECHNIQUE STEPS AND POST-PROCEDURAL QCA PARAMETERS
Predilation was not associated with better post-procedural QCA values. Correct scaffold size was significantly associated with higher post-procedural MLD and lower maximum footprint. Post-dilation using a non-compliant balloon larger than the BRS diameter up to a maximum of 0.5 mm (definition of PSP-1 and PSP-3) was associated with lower maximum footprint, but not with post-procedural MLD (Supplementary Table 7).
IMPLANTATION TECHNIQUE AND 1-YEAR CLINICAL EVENTS
During the one-year follow-up period, seven cases of DoCE and two cases of ScT were observed. There was no significant difference in terms of DoCE between optimal and non-optimal BRS implantation according to PSP-1 (1.8% vs. 1.6%, p=1.000), PSP-2 (1.4% vs. 1.6%, p=1.000), and PSP-3 (0% vs. 1.7%, p=1.000). The incidence of ScT was also comparable between optimal and non-optimal BRS implantation at one year (0% vs. 0.5%, p=1.000 for PSP-1, PSP-2, and PSP-3).
Discussion
The main findings of this study on STEMI patients undergoing primary PCI are the following. 1) Optimal BRS implantation is significantly associated with better post-procedural QCA parameters, in particular with higher post-procedural MLD and lower maximum scaffold footprint. 2) Manual thrombectomy is an independent predictor of better post-procedural QCA measurements. A combination of thrombectomy with optimal BRS implantation exhibits a trend towards higher MLD and lower maximum footprint as compared to conventional optimal or non-optimal BRS implantation. 3) Correct scaffold sizing and post-dilation using an adequate non-compliant balloon size were key steps to achieving good post-procedural QCA parameters after BRS implantation in STEMI patients.
Recent analyses have shown a higher rate of adverse clinical events with BRS as compared with metallic drug-eluting stent implantation11,12. Optimisation of BRS implantation has been advocated as a possible solution to reduce adverse events7,13. Post-procedural QCA parameters have also been related to adverse clinical events6. For these reasons, our analysis focused on the impact of BRS implantation technique on QCA parameters, which can be considered as surrogate endpoints for adverse clinical events.
We showed that optimal BRS implantation, coded as maximum PSP score, is indeed related to better post-procedural MLD and maximum footprint. The BVS STEMI STRATEGY-IT study was designed with the aim of providing an “easy” chart (no mandatory on-line QCA or intracoronary imaging guide) to drive optimal BRS implantation in STEMI patients. However, among patients, only 8 to 14% underwent optimised BRS implantation according to PSP parameters. This rate is consistent with the previous study7. The low compliance to PSP technique in this study may stem from the fact that correct scaffold sizing by visual estimation and appropriate post-dilation were accomplished in a limited proportion of patients (21.5% and 56.8%, respectively). This could be a consequence of the fact that the PSP technique has been developed in an all-comers population, but it may not be totally applied in a STEMI population, who may need a different optimised BRS implantation technique. A conventional PSP strategy may indeed be difficult to achieve in the STEMI setting because predilation and/or post-dilatation (using a non-compliant balloon larger than the nominal diameter of the BRS) are not routinely recommended to avoid distal embolisation due to thrombus or soft plaque14. Moreover, coronary vasoconstriction, despite the administration of nitrates, may interfere with adequate vessel and scaffold visual sizing15.
An interesting finding of our analysis is the role of thrombectomy in BRS implantation for STEMI. Despite the lack of a mortality impact, thrombectomy has been reported to allow implantation of larger stents in primary PCI16. By reducing the amount of thrombus, thrombectomy may help to achieve a more accurate sizing and may also reduce the risk of distal embolisation during balloon dilation17. In our analysis, we found, for example, that patients with thrombectomy received a larger scaffold (3.5 mm vs. 3.0 mm, p=0.041) than patients without thrombectomy. The combination of thrombectomy and optimal BRS implantation exhibits a trend towards a higher MLD and a lower maximum footprint. A future study should be carried out to clarify the efficacy of a thrombectomy-PSP strategy in STEMI patients.
Our analysis has also demonstrated that correct scaffold sizing and post-dilation were key steps in BRS implantation in order to achieve better post-procedural QCA parameters in STEMI patients. Because BRS have an upper limit of expansion, correct scaffold sizing is essential in order to avoid disruption, underexpansion or malapposition18. Whereas BRS undersizing causes malapposition, BRS oversizing may be associated with dissection and adverse events19. Post-dilation using a non-compliant balloon larger than the BRS diameter up to a maximum of 0.5 mm was also associated with better post-procedural QCA parameters. Although post-dilation was not statistically associated with post-procedural MLD as a continuous variable, it was instead significantly associated with high post-procedural MLD categorised as a binary variable (high/low), using the definitions of Puricel et al6 (47.0% vs. 36.1%, p=0.022). A recent study demonstrated that routine post-dilation was not associated with larger MLD after BRS implantation in a STEMI population20. However, in that study, approximately 80% of the patients underwent post-dilation using a balloon smaller than the BRS diameter or larger than 0.5 mm above the nominal BRS diameter. This may explain the difference between our results and those findings. Even with achievement of angiographic success, aggressive post-dilation using an adequate balloon size was associated with good BRS expansion with a low incidence of malapposition and edge dissection, confirmed by OCT analysis21. Therefore, mandatory post-dilation using an adequate non-compliant balloon size (>1:1 balloon-to-artery ratio) may be necessary for BRS implantation in STEMI patients.
Optimal BRS implantation was not associated with higher acute gain in multivariate analysis (Supplementary Table 8). Among patients without optimal BRS implantation, more than 80% received an oversized BRS; this may explain the lack of difference in acute gain between optimal vs. non-optimal BRS implantation.
Finally, although our study demonstrated a relationship between optimal BRS implantation and post-procedural QCA analysis parameters in STEMI, no relationship between optimal BRS implantation and clinical events was observed in the one-year follow-up data of BVS STEMI STRATEGY-IT. Because only seven cases of DoCE and two cases of ST occurred at one-year follow-up, it might be difficult to assess correctly the impact of QCA parameters and optimal BRS implantation on adverse events. Therefore, this requires to be investigated in the future with a follow-up longer than one year. Although the Absorb everolimus-eluting BRS is not available anymore due to its withdrawal from sale, a specific BRS implantation technique may be used for any kind of bioresorbable device intended to be implanted in STEMI patients.
Limitations
This study has several limitations. First, this is a retrospective analysis of a prospective multicentre registry. Second, about 10% of patients were excluded because of an inadequate angiogram for QCA analysis. However, the clinical and procedural characteristics were not different between included and excluded patients. Third, though the pre-specified BRS implantation strategy might be associated with lesion characteristics, data on lesion characteristics, particularly the presence of severe calcification, were lacking in our analysis. However, the proportion of severe calcification in STEMI patients was reported to be relatively low22, and therefore our results might not have changed. Fourth, because the incidence of adverse events was low during the initial one-year follow-up (seven cases of DoCE and two incidences of definitive or probable ScT), the relationship between clinical endpoints and optimal BRS implantation cannot be assessed. Longer follow-up and a larger number of patients are required to verify the relationship between the post-procedural QCA data and clinical events after Absorb BRS implantation in patients with STEMI.
Conclusions
In STEMI patients, optimal BRS implantation, assessed by the PSP score, was associated with higher post-procedural MLD and lower maximum footprint. Manual thrombectomy was an independent predictor of better post-procedural QCA parameters. At one-year follow-up, no relationship was observed between optimal BRS implantation and incidence of adverse events. Long-term follow-up is warranted to investigate further the relationship between these post-procedural QCA parameters and clinical outcomes after BRS implantation in STEMI patients. The use of a modified PSP technique, including thrombectomy, also deserves further investigation.
|
Impact on daily practice Optimal BRS implantation, assessed by the maximum PSP scores, was significantly associated with higher post-procedural MLD and lower maximum scaffold footprint in STEMI patients. In particular, correct scaffold size and post-dilation were key steps to obtaining better post-procedural QCA parameters. Manual thrombectomy prior to BRS implantation may play an important role in achieving better post-procedural QCA parameters in STEMI patients. |
Acknowledgements
The authors thank the investigators and institutions that have participated in the BVS STEMI STRATEGY-IT study.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Pre-specified BRS implantation flow chart. Supplementary Table 1. PSP score model.
Supplementary Table 2. Differences in patient characteristics between included and excluded patients.
Supplementary Table 3. Patient characteristics according to the maximum PSP-1.
Supplementary Table 4. Patient characteristics according to the highest PSP-2.
Supplementary Table 5. Patient characteristics according to the maximum PSP-3.
Supplementary Table 6. Multivariate analysis for predictors of low maximum scaffold footprint.
Supplementary Table 7. Implantation technique and post-procedural QCA data.
Supplementary Table 8. Multivariate analysis for predictors of acute absolute gain.

