Abstract
The Absorb bioresorbable scaffold (BRS), the most studied device among all BRS, suffered a major setback following the negative results of the ABSORB trials. However, approximately 34 BRSs from 22 companies are currently under development. The potential device-specific factors related to the increased event rate in Absorb were: 1) weak mechanical properties, 2) larger strut thickness (less embedment and larger protrusion) and width (larger footprint) predisposing to underexpansion/protrusion of struts, eventually resulting in increased thrombogenicity, and 3) longer bioresorption time combined with failure of encapsulation of struts before the dismantling process ensues. Given the diversity of bioresorbable materials (even amongst PLLA), and the different mechanical properties and bioresorption profiles of each new BRS, one could expect considerable difference in early and late clinical outcomes. As a matter of fact, data from first-in-man (FIM) and pivotal trials have demonstrated variable clinical results. Indeed, early clinical evidence from FIM trials does not support a class effect. However, the absence of a comparator precludes us from drawing definitive conclusions. Further clinical evidence should confirm the absence (or presence) of a class effect.
Abbreviations
| BRS | bioresorbable scaffold |
| DES | drug-eluting stent |
| EES | everolimus-eluting stent |
| FIM | first-in-man |
| MACE | major adverse cardiac events |
| OCT | optical coherence tomography |
| PCI | percutaneous coronary intervention |
| PLLA | poly-L-lactic acid |
| RCT | randomised controlled trial |
| ScT | scaffold thrombosis |
| TLR | target lesion revascularisation |
| VLScT | very late scaffold thrombosis |
Introduction
Following the promising results of the ABSORB cohort B study1, the ABSORB II randomised controlled trial (RCT) was conducted to test the Absorb™ bioresorbable scaffold (BRS; Abbott Vascular, Santa Clara, CA, USA) against the best-in-class metallic drug-eluting stent (DES) as a comparator. However, the Absorb suffered a major setback when the results of the co-primary endpoints did not meet the hypothesis. Namely, quantitative differences in vasomotion were not observed between the devices, and late loss in the Absorb BRS was significantly larger than in the XIENCE stent (Abbott Vascular)2. In addition, the device-oriented composite endpoint (cardiac death, target vessel myocardial infarction, and clinically indicated target lesion revascularisation [TLR]) at three years was higher in Absorb than in XIENCE (10% vs. 5%, p=0.0425). There were nine cases of definite/probable scaffold thrombosis (ScT) in the Absorb, whereas no stent thrombosis was observed in the XIENCE (p=0.0331).
Furthermore, the three-year results of the ABSORB III trial showed that the rate of target vessel myocardial infarction was higher in the Absorb BRS (8.6% vs. 5.9%; p=0.03), as was the rate of device thrombosis (2.3% vs. 0.7%; p=0.01), compared to the XIENCE metallic stent3.
In addition, in the Amsterdam Investigator-Initiated Absorb Strategy All-Comers Trial (AIDA), which randomised patients undergoing PCI to receive either the Absorb scaffold (924 patients) or the XIENCE metallic stent (921 patients)4, although the rate of target vessel failure was not significantly different (11.7% vs. 10.7%, p=0.43), definite or probable device thrombosis occurred more frequently in the Absorb group as compared with the XIENCE group (3.5% vs. 0.9%, p<0.001). Accordingly, the manufacturer decided on a worldwide halt to sales of the scaffold as of September 2017.
The early enthusiasm has been tempered following the negative results of the ABSORB trials; however, approximately 34 BRSs from 22 companies are currently under development (Supplementary Table 1). All BRSs are developed under the common concept that they “provide short-term vessel support and inhibit early constrictive remodelling and disappear with the resorption process in-between”. However, given the considerable differences in mechanical properties, absorption process, and drugs eluted, it is currently unclear whether all BRSs have a class effect5.
Generally, a class effect refers to an effect produced by all members of the group and not only by a single element from that class. However, there is no standard definition of “class effect”. Instead, a related term, “class labelling”, is used by the Food and Drug Administration (FDA). Class labelling “assumes that all products within a class are closely related in chemical structure, pharmacology, therapeutic activity, and adverse reactions”6.
The aim of this review is to consider whether we may extrapolate the failure of Absorb to all the other BRSs as a “class effect” based on limited information from first-in-man (FIM) and pivotal trials concerning the different BRSs available so far. First, the presumed mechanisms of the scaffold failure and mechanical properties in BRSs are discussed. Second, angiographic and clinical outcomes in the FIM/pivotal trials are reviewed, where acute recoil, late loss, and ischaemia-driven TLR were selected as efficacy endpoints, and target vessel myocardial infarction and ScT as safety endpoints. Third, we summarise the data and discuss future perspectives.
PRESUMED MECHANISMS OF THE SCAFFOLD FAILURE
In a meta-analysis of patients treated with BRS (n=3,261) and EES (n=2,322), the significant difference in the two-year device-oriented composite endpoint between BRS and EES was no longer seen after exclusion of device thrombosis cases, suggesting a large impact of ScT on scaffold failure7.
There are several presumed specific mechanisms of the increased event rates with BRS. In cases with early ScT, mechanical factors such as scaffold underexpansion, undersizing, or geographical miss and insufficient platelet inhibition were reported as possible causal factors8. On the other hand, as underlying causes of very late scaffold thrombosis (VLScT), scaffold discontinuity, malapposition, neoatherosclerosis, underexpansion or scaffold recoil, uncovered struts, and edge-related disease progression were reported from the INVEST registry9.
Generally, bioresorbable materials are mechanically less strong (discussed in the next section). Scaffolds need to have thicker struts and a larger footprint than metallic stents to maintain comparative mechanical strength. Lower radial strength causes underexpansion and wider struts result in less embedment10.
In the early phase, mechanical factors such as underexpansion and less embedment cause disturbed microcirculation. In a flow simulation of a microenvironment computed by optical coherence tomography (OCT)/angiography fusion in a human coronary artery, the relatively high endothelial shear stress on top of the strut and low endothelial shear stress measured behind and between the thick BRS struts were demonstrated11. High shear stress on top of thick struts promotes platelet activation and release of adenosine diphosphate and thromboxane A2, two potent platelet agonists12. Conversely, recirculation zones with low endothelial shear stress downstream of the strut increase the local concentration of activated platelets at the site of denuded endothelium in the absence of production of antithrombotic factors including nitric oxide and prostacyclin. In addition to microcirculation disturbance, scaffold material has been shown to influence thrombogenicity. It was highlighted in a report by Waksman et al13 that magnesium scaffolds were less thrombogenic than poly-L-lactide (PLLA) scaffolds or metallic DES in a porcine arteriovenous shunt model.
In a later phase, in the presence of relevant areas of malapposed or uncovered scaffold struts, late scaffold discontinuity may cause dislocation of thrombogenic strut remnants into the lumen, leading to disturbed haemodynamic flow and activation of the thrombotic cascade that can potentially result in VLScT14. In addition, neoatherosclerosis five years after BRS implantation was reported15, challenging the concept of plaque sealing by BRS. It should be noted, however, that the study15 needs to be interpreted with caution since it lacks a comparator device.
MECHANICAL PROPERTIES
Generally, bioresorbable materials have a stiffness and tensile strength considerably lower than those of permanent metals (Figure 1, Supplementary Table 2). The stiffness and tensile strength of a material are linked to the radial force of the scaffold, and the strain at break limits the allowable dilatation diameter. To that effect, the most prominent difference compared to permanent stents is the strut thickness (Figure 2). Ideally, the mechanical support provided by BRS in the first few months should be as good as that provided by metallic stents. In a simulated bench test, magnesium-based BRSs matched the recoil characteristics and radial strength of permanent metal stents, but larger strut dimensions were required to achieve this16. Polymeric materials, showing a stiffness and tensile strength considerably lower than those of magnesium, require even more effort in terms of dedicated scaffold designs.
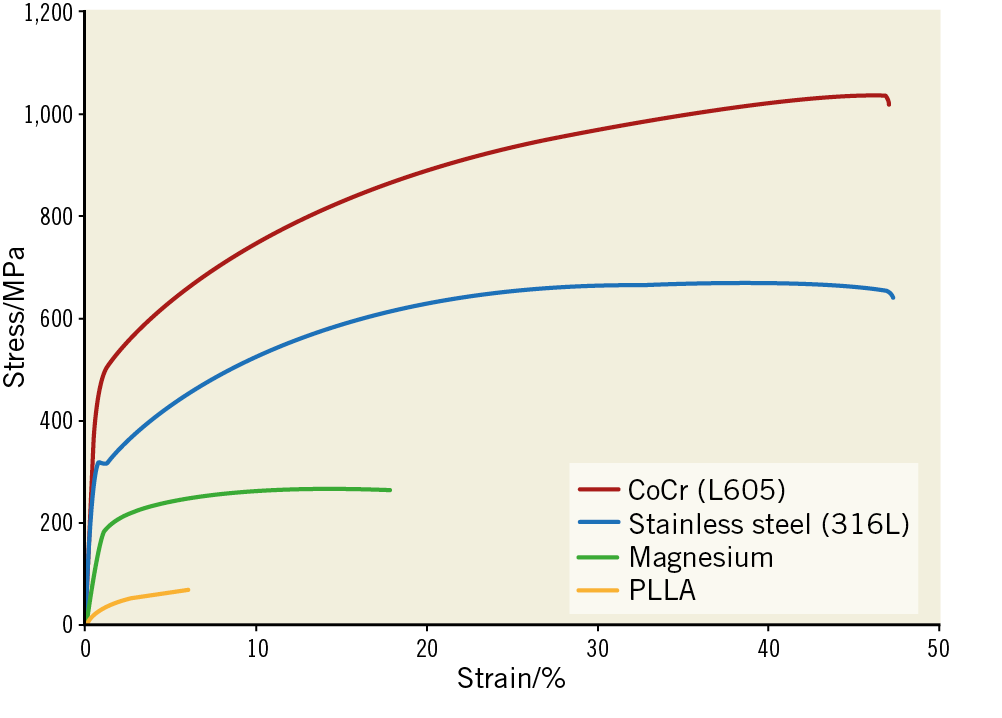
Figure 1. Exemplary engineering stress-strain curves of various materials used for vascular implants. The graphs compare the mechanical behaviour of different commonly used materials. Reproduced from Lootz et al29, with permission.
In addition, it is remarkable that the mechanical properties can change in a time-dependent manner. In the acute phase, the in vitro study by Schmidt et al17 showed that the elastic recoil of the Absorb scaffold immediately after balloon deflation (5.9%) is comparable to that of the Magmaris™ (Biotronik, Berlin, Germany) (5.6%) but increases to 7.0% after one hour due to relaxation of the polymer, whereas the elastic recoil of the Magmaris does not change over time, remaining in the range of permanent metallic stents (2-6%)18. DESolve® (Elixir Medical Corp., Milpitas, CA, USA) showed high elastic recoil of nearly 8% immediately after balloon deflation but, after one hour, it increases its diameter by 3.0% beyond the initial dilated diameter17, reproducing the finding termed “self-correction” by Ormiston et al19. These large variabilities in the intrinsic properties of the materials that devices are made of make the class effect highly unlikely.
At a later phase, mechanical properties could interact with biodegradation. Scaffold degradation could weaken the radial force over time, and could be the main cause of vessel recoil and restenosis20,21, especially in magnesium, which biodegrades in one year.
Angiographic and clinical outcomes
ACUTE RECOIL
In FIM trials, acute recoil of BRSs was higher than that of metallic stents, except for the Fantom® scaffold (REVA Medical, San Diego, CA, USA) made of desaminotyrosine polycarbonate (Table 1). Acute recoil of PLLA scaffolds ranged from 4.3 to 6.7%. Although in vivo acute recoil in magnesium BRSs is not available, a result from bench testing suggests high acute recoil despite a thickness of 150 µm.
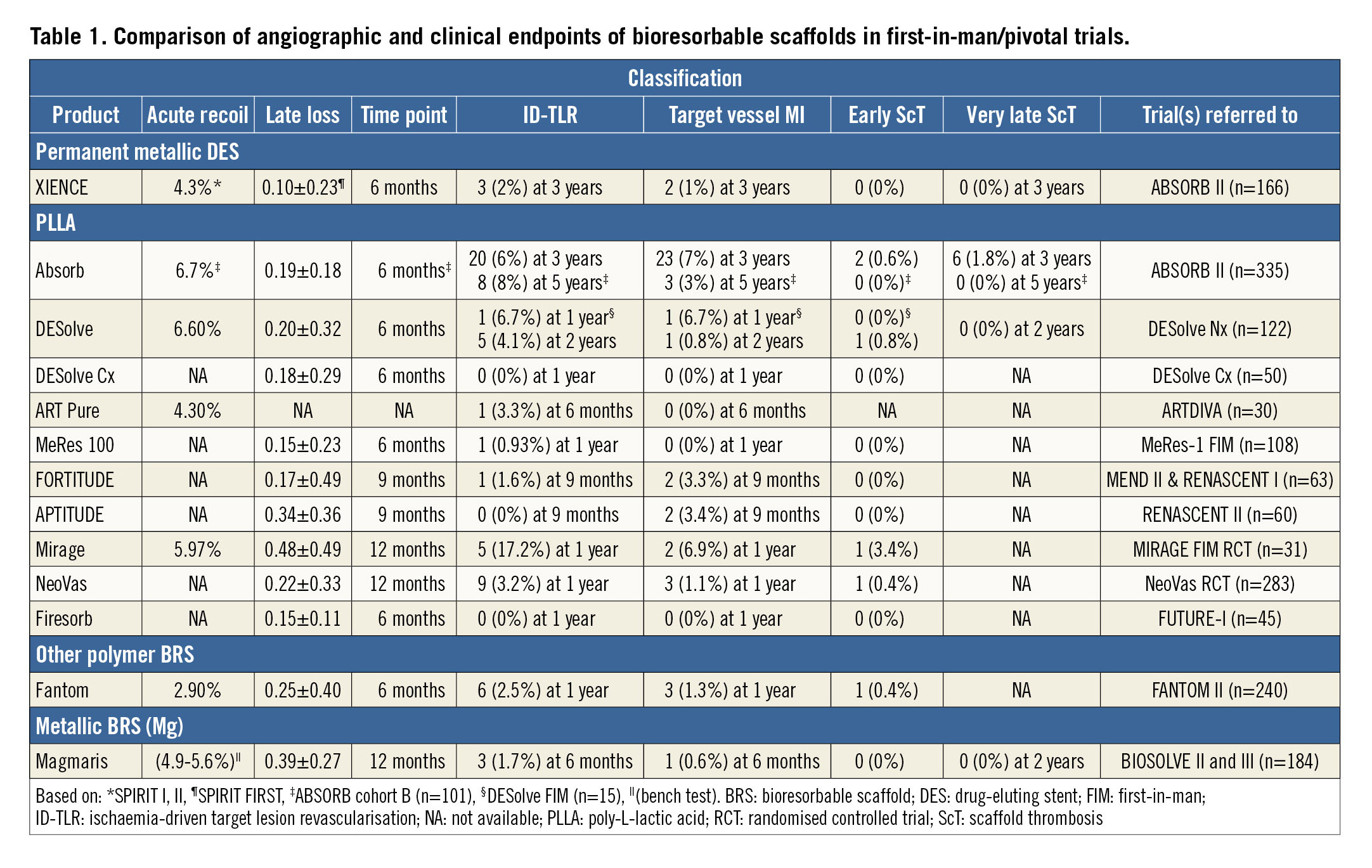
LATE LOSS AND INCREASED TLR
Late loss up to 12 months in FIM trials is shown in Table 1. None of the BRS achieved less late loss as compared to XIENCE. Even among PLLA scaffolds, late loss ranged from 0.15 to 0.48 mm. The lowest late loss among BRS was observed in a scaffold with the thinnest strut, MeRes100™ (Meril Life Sciences, Vapi, India) whereas the highest late loss was observed in the Mirage PLLA scaffold (Manli Cardiology, Singapore). Although the strut thickness of the Mirage is 125 µm, its high strut/vessel coverage and unique structure (i.e., overlapping fibre configuration) might have influenced late loss. The lowest acute recoil in the Fantom scaffold did not translate into the lowest late loss. Most BRSs elute sirolimus (Figure 2); however, the drug itself and drug release may play an important role in late loss.
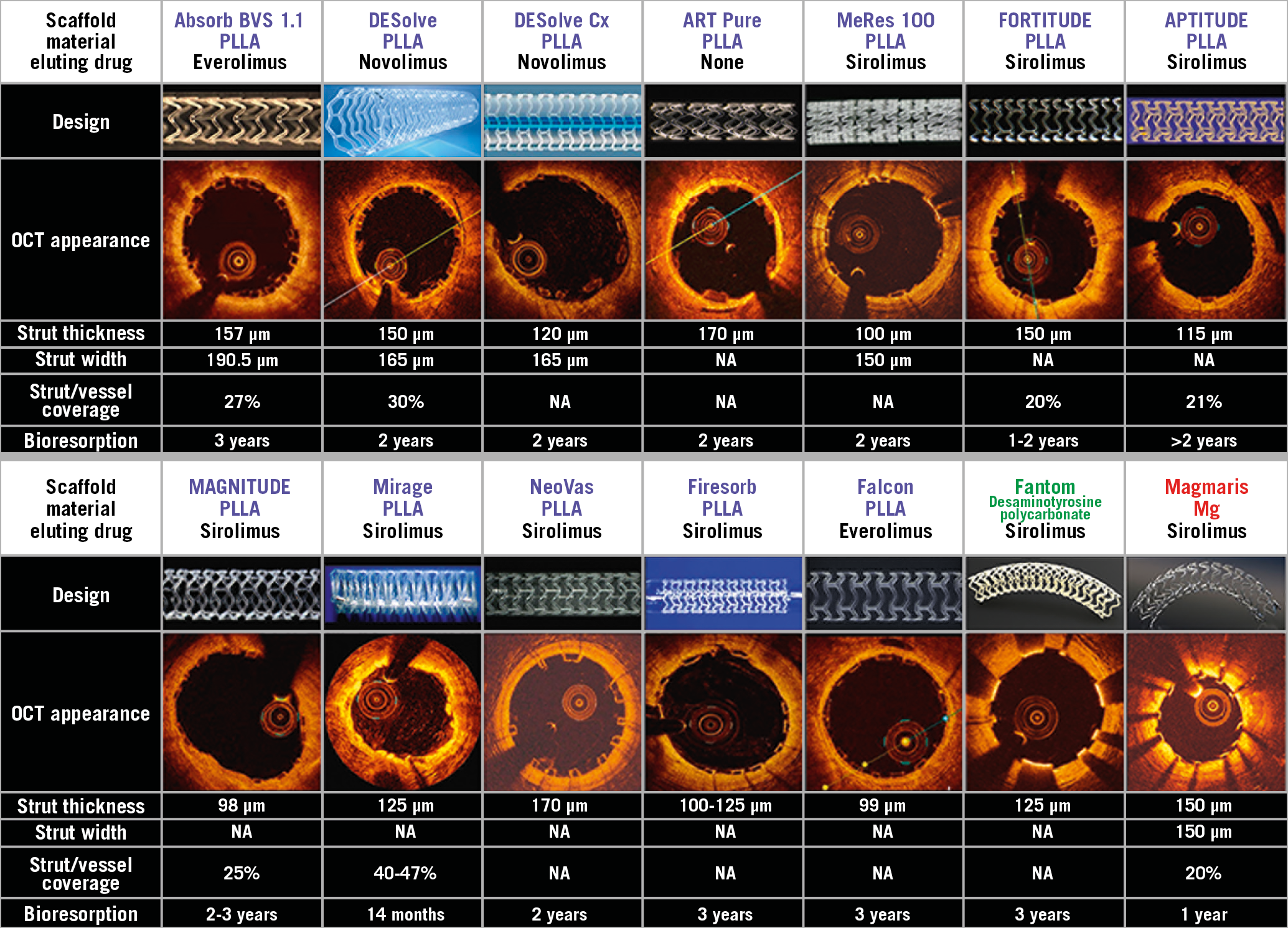
Figure 2. Key characteristics and OCT cross-sectional images of bioresorbable scaffolds. NA: not available; PLLA: poly-L-lactic acid
TLR usually reflects restenosis or thrombotic occlusion. It also varies per device, even amongst PLLA products. The MeRes 100 and Firesorb (MicroPort, Shanghai, China) PLLA scaffolds showed low TLR rates (0.93% and 0% at one year, respectively), with the lowest late loss being 0.15 mm at six months. In contrast, the Mirage demonstrated the highest TLR rate among BRSs with a high late loss of 0.48 mm at 12 months. Apparently, from these observations, late loss correlates with TLR, as reported previously22. However, while Absorb, DESolve, and DESolve Cx had similar late loss at six months (0.19, 0.20 and 0.18 mm, respectively), the TLR rate varied − 6% at three years, 6.7% at one year, 0% at one year in each device, respectively. This fact could suggest that the difference in TLR cannot be explained solely by late loss. Differences in configuration or drug elution profile, as well as implantation technique, may play a role as predicting factors of the diverse rates of TLR13,23.
TARGET VESSEL MYOCARDIAL INFARCTION
The rate of target vessel myocardial infarction varies among BRS (Table 1). Target vessel myocardial infarction in Absorb, DESolve and Mirage was observed in ≥6% of patients at 3, 1, and 1 years, respectively. In contrast, ART Pure (Arterial Remodeling Technologies, Paris, France) and NeoVas™ (Lepu Medical Technology, Beijing, China) had low rates of target vessel myocardial infarction although these two devices have the thickest struts (170 µm) (Figure 2). However, the percentage from a study with a small number of patients needs to be interpreted with caution.
EARLY SCAFFOLD THROMBOSIS
The rates of early ScT were generally low in BRS as well as in metallic stents in well selected patient populations and simple lesions enrolled in FIM/pivotal trials (Table 1). In BRSs, ScT rates varied from 0 to 0.8%, excluding 3.4% of ScT in the MIRAGE FIM RCT considering its small population.
VERY LATE SCAFFOLD THROMBOSIS
The fact that there are few studies with follow-up >1 year makes a comparison of VLScT rates among BRSs difficult. In addition, DAPT duration can be a confounding factor. However, Absorb and DESolve, although both are made of PLLA, have a large difference in the nominal rate of VLScT, 1.8% and 0%, respectively (Table 1).
Summary and future perspectives
IS THE FAILURE OF ABSORB APPLICABLE TO OTHER BRSs?
With the Absorb scaffold, the potential device-specific factors related to increased events were: 1) a less strong mechanical property, 2) a larger strut thickness and footprint which can predispose to underexpansion/protrusion of struts, eventually resulting in increased thrombogenicity, 3) bioresorption time and failure of strut encapsulation before dismantling.
According to the 2018 ESC/EACTS Guidelines on myocardial revascularisation, BRS are currently not recommended for clinical use outside of clinical studies (Class III, Level C)24. However, the question remains whether the failure of Absorb is applicable to other BRSs due to the following facts. 1) Mechanical properties vary with each bioresorbable material (even amongst PLLA by post-processing of the polymer). 2) New devices have thinner struts or a smaller footprint. Thrombogenicities of various materials are different (e.g., magnesium scaffold). 3) Each device has a unique biodegradation profile (bioresorption duration ranging from one to three years). These facts suggest that each BRS is different and that there would be no class effect. As a matter of fact, FIM/pivotal trial data have demonstrated various clinical results. Therefore, careful consideration should be given before making a general recommendation of BRS as a “class”.
HOW CAN WE ADDRESS LATE SCAFFOLD DISCONTINUITY?
Thinner struts with deeper embedment and less protrusion would allow early encapsulation of struts by tissue, which may prevent late discontinuity. The next-generation Absorb BRS, “Falcon”, has a strut thickness of 99 µm (Figure 2) and is currently in the preclinical phase (Supplementary Table 1).
The current limitations of PLLA could be overcome by a post-processing technique. Tensile strength and radial force can be increased by post-processing, altering the molecular orientation of polymer or increasing molecular weight. Through a heating and extrusion process, undrawn semicrystalline polymer can become oriented and stronger structures are created25. Previous studies have shown that the PLLA-based BRS platform of Amaranth Medical (Mountain View, CA, USA), through a proprietary process of ultrahigh molecular weight polymer synthesis and processing, shows elongation at break points 10 times higher compared to currently used PLLA. The company is trying to reduce the strut thickness from 150 μm in the FORTITUDE®, to 115 μm in the APTITUDE®, and to 98 μm in the MAGNITUDE® BRS (Figure 2). The former two iterations had no ScT at nine months. The MAGNITUDE BRS is being tested in the RENASCENT III trial (n=70). However, the efficacy of these various post-processing methods has not yet been confirmed in the clinical arena.
The effort to find the best material is also of importance as the duration of bioresorption differs among materials. Most BRS polymers absorb by bulk erosion, with the surface and interior of the material degrading at similar rates, a non-enzymatic process, which for most polymers is controlled mainly by temperature and water concentration. In contrast, most metallic BRS (magnesium and iron alloys) degrade (“corrode”) by surface erosion.
Prevention of malapposition by either a BRS-specific implantation strategy26, or OCT-guided implantation, and new-generation BRS with thinner struts could contribute to early neointimal coverage and a consequent reduction of the incidence of late ScT and VLScT.
DUAL ANTIPLATELET THERAPY AFTER IMPLANTATION OF BIORESORBABLE SCAFFOLDS
Treatment with dual antiplatelet therapy (DAPT) after BRS implantation is mandatory to mitigate the risk of ScT27. However, the optimal duration of DAPT treatment is unknown. Thicker and wider BRS struts might cause a higher risk of stent thrombosis, as compared to DES with thinner struts2. Moreover, thicker stent struts may take longer to be completely covered by neointima. Importantly, due to concerns regarding VLScT in the course of scaffold degradation at two to three years, it is conceivable that the duration of DAPT treatment may need to be prolonged to the time of BRS bioresorption28. Currently, a longer duration of DAPT is recommended, at least in patients at low bleeding risk.
WHAT KIND OF CLINICAL STUDY DO WE NEED?
Although the event rates observed in the ABSORB cohort B were considered to be acceptable in the absence of comparators, the Absorb BRS was inferior in terms of angiographic and clinical endpoints in the ABSORB II RCT.
The ESC-EAPCI Task Force suggests evaluation of current and future devices according to a standard plan (Figure 3),28. Initial human feasibility studies with BRS should be small-sized (N=50-150) in selected patients. These studies may be planned as single-arm, prospective, observational studies. The aim is to support the claim of efficacy and safety but also to assess vessel-device interactions and the bioresorption process. In this regard, angiographic and intravascular imaging assessment should be performed at baseline, at six to twelve months, and at the time of complete resorption.
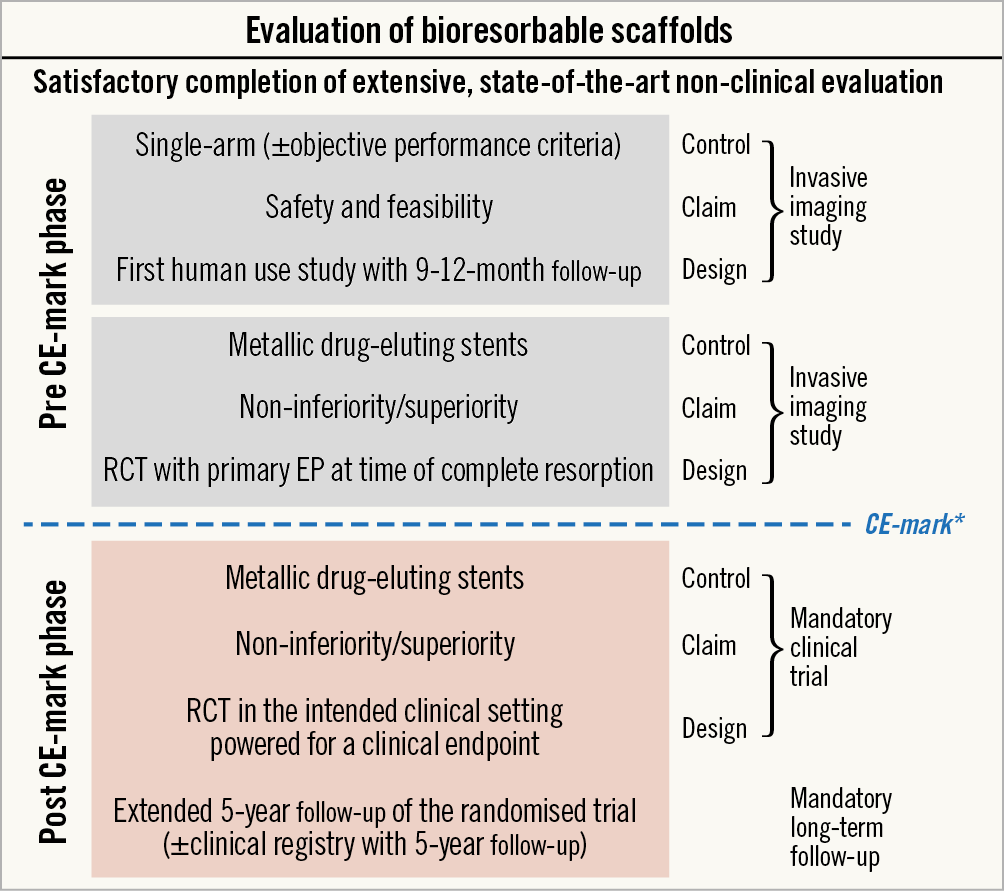
Figure 3. Task Force recommended clinical evaluation plan for bioresorbable scaffolds. *The manufacturer must submit and have approved by the notified body a plan for post-market clinical follow-up in the form of a large-scale, randomised trial±a large-scale clinical registry. Reproduced from Byrne et al28, with permission. EP: endpoint; RCT: randomised controlled trial
Subsequently a medium-sized, randomised trial (N=200-500) should be undertaken, powered for the detection of differences in surrogate endpoints in comparison with comparator devices. This should be based on angiographic follow-up at six to 12 months and include intracoronary imaging in a subgroup of patients (N=50-100) to compare arterial healing response. Comparator devices should be contemporary metallic DES. As a minimum requirement, these steps should be completed with satisfactory results before CE mark approval of any new BRS.
Subsequently, comparative efficacy testing against a benchmark DES in a trial powered for a device- or patient-oriented outcome (N=1,500-2,500) is recommended. A non-inferiority design for the assessment of outcomes within one year would be acceptable, but sequential designs followed by superiority during longer-term follow-up (three to five years) are recommended in order to evaluate the long-term effects of BRS, although this depends on the specific bioresorption profile of any given device.
Given large variabilities in the intrinsic properties of the materials that devices are made of, every device should be evaluated individually as recommended by ESC-EAPCI guidelines for BRS evaluation28.
Limitations
When considering the presumed mechanism of scaffold failure, we have to rely on the data from Absorb BRS, since, to the best of our knowledge, the Absorb is the most studied BRS so far. In the absence of serial intravascular imaging, the mechanism of scaffold failure could not be fully elucidated. The necessity of an adequate number of control patients well matched with event cases is another challenging issue in the setting of an OCT registry. In addition, the results of a FIM trial cannot necessarily be translated to the results of later clinical trials, as was the case in the ABSORB trials. Considering that the common denominator of the BRS is the requirement of full absorption, a “class effect” after scaffold resorption may be present at long-term follow-up. To elucidate the complete picture of clinical results after implantation of BRS, longer-term follow-up after complete bioresorption is needed.
Conclusions
Considering the diversity of bioresorbable materials (even amongst PLLA) in terms of mechanical properties and bioresorption profiles, each BRS could have differences in early and late clinical outcomes. This suggests that there is no class effect in BRS. However, early clinical data are inadequate to respond fully to this question in view of sample size, simple lesions involved, and learning curve. Further clinical evidence should confirm the absence (or presence) of a class effect.
|
Impact on daily practice Given the diversity of bioresorbable materials, and the different mechanical properties and bioresorption profiles of each new BRS, considerable differences are expected in early and late clinical outcomes. As a matter of fact, data from first-in-man and pivotal trials have demonstrated variable clinical results. Careful consideration should be given before making a general recommendation of BRS as a “class”. |
Guest Editor
This paper was guest edited by Adnan Kastrati, MD; Deutsches Herzzentrum, Munich, Germany.
Conflict of interest statement
Y. Onuma is a member of the Advisory Board of Abbott Vascular. P.W. Serruys reports consultant fees from Abbott, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sinomedical Sciences Technology, Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems, Volcano, St. Jude Medical, and Qualimed. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. List of bioresorbable scaffolds and development status.
Supplementary Table 2. Material properties of stent and scaffold materials.

