Valvular heart disease and gastrointestinal bleeding (GIB) due to angiodysplasia have a well-established link with aortic stenosis, where GIB is believed to be mediated by increased shear stress and acquired von Willebrand (VW) syndrome12. Platelet dysfunction and VW factor abnormalities are less commonly described in mitral regurgitation3 and in dysfunctional valve prostheses4. In these cases, valve replacement frequently solves abnormalities in primary haemostasis14. Little is known about the relationship between mitral paravalvular leaks (PVL) and GIB due to angiodysplasia and whether PVL closure affects the natural history of GIB in these patients. We hypothesised that high velocity jets through the PVL orifice could induce gastrointestinal bleeding due to intestinal angiodysplasia that may otherwise improve if PVL closure is achieved (Figure 1A).
To assess the relationship between PVL and GIB, we included in our study all percutaneous closures of mitral PVL performed in symptomatic patients at a tertiary referral centre between January 2010 and November 2022. Follow-up was completed in May 2023. Redo procedures were not included. To assess the impact of procedural success in recurrent GIB, patients were divided into two groups based on the transoesophageal echocardiographic result of PVL closure. Procedural success was defined as a residual PVL ≤mild. Recurrent GIB was defined as two or more episodes of GIB causing a decrease in haemoglobin ≥2 g/dl or GIB requiring a transfusion of ≥2 units of red blood cells, and it was analysed separately, both before and after percutaneous mitral PVL closure. Fisher’s exact test was used to compare the rates of patients with recurrent bleeding according to procedural success. The study was approved by the local institutional review board (PR[AG]635/2021).
During the study period, 96 patients underwent percutaneous closure of mitral PVL (Central illustration). The median age of patients was 73 (interquartile range [IQR] 66-78) years, and 51 patients (53.1%) were female. The main indications for PVL closure included heart failure (HF) in 37 patients (38.5%), haemolysis in 11 (11.5%), and both conditions in 48 (50.0%). All closures were performed on surgically placed prosthetic mitral valves, of which 80 (83.3%) were mechanical, 78 (81.3%) had severe mitral PVL, 39 (40.6%) had multiple defects, and 52 (54.2%) required more than 1 closure device. Almost all patients (97%) were on oral anticoagulants, and 32 (33%) had an estimated glomerular filtration rate <60 ml/min/1.73 m2. During the year preceding PVL closure, 11 (11%) patients had major recurrent GIB, with angiodysplasia diagnosed in 10 of them. There were no significant differences in terms of the severity or the presence of multiple leaks, nor in terms of anticoagulant treatment between patients with or without GIB before PVL closure. Among these 11 patients, PVL closure was successful in 6 patients, while the other 5 patients continued to experience residual PVL >mild. Procedural success in these patients was associated with lower rates of recurrent GIB (0% vs 80%; p=0.015); thus, none of the patients in whom PVL closure was successful experienced recurrent GIB. In contrast, 4 of the 5 patients whose procedure had failed subsequently developed recurrent GIB during the follow-up period; the fifth patient died shortly after the intervention due to refractory heart failure. The median time to the first GIB was 3 (IQR 1-19) months, and patients had a median of 3 GIB episodes during the follow-up period. GIB-related death occurred in 3 of the 5 patients with unsuccessful PVL closure (Figure 1B). Overall, recurrent GIB after PVL closure was observed in 7 out of 96 patients (7.2%). The mean follow-up period was 3.5±3.3 years. Of the 7 patients with recurrent GIB after PVL closure, 3 had not previously experienced GIB. Of these 7 patients, all (100%) had a residual leak >mild, while procedural failure was observed in 43% of those without recurrent GIB (p=0.004). Overall, there were 14 patients with recurrent GIB either before or after PVL closure, and angiodysplasia was confirmed in 12 (86%) of them using upper and lower endoscopy (all patients) and capsule endoscopy (n=9 patients).
There are some noteworthy considerations that arise from this study. First, the proportion of angiodysplasia in patients with recurrent GIB and PVL (86% in our study) is notably higher than the 5-10% reported in an all-comers study5, suggesting a potential relationship between the two conditions. Second, the significant association between procedural failure and GIB relapse suggests that patients with recurrent GIB related to intestinal angiodysplasia may benefit from PVL closure, aside from the benefits in terms of haemolysis or HF. Lastly, unsuccessful PVL closure may favour the onset of de novo recurrent GIB.
Several limitations of our study, including the retrospective design, small sample size, and potential confounding factors such as anticoagulant therapy, could introduce bias, limit generalisability, and hinder the establishment of causal relationships.
In conclusion, GIB related to angiodysplasia may prompt the evaluation of PVL in carriers of prosthetic mitral valves, as successful PVL closure may resolve GIB episodes. However, further studies are needed to consider recurrent GIB as an indication for mitral PVL closure.
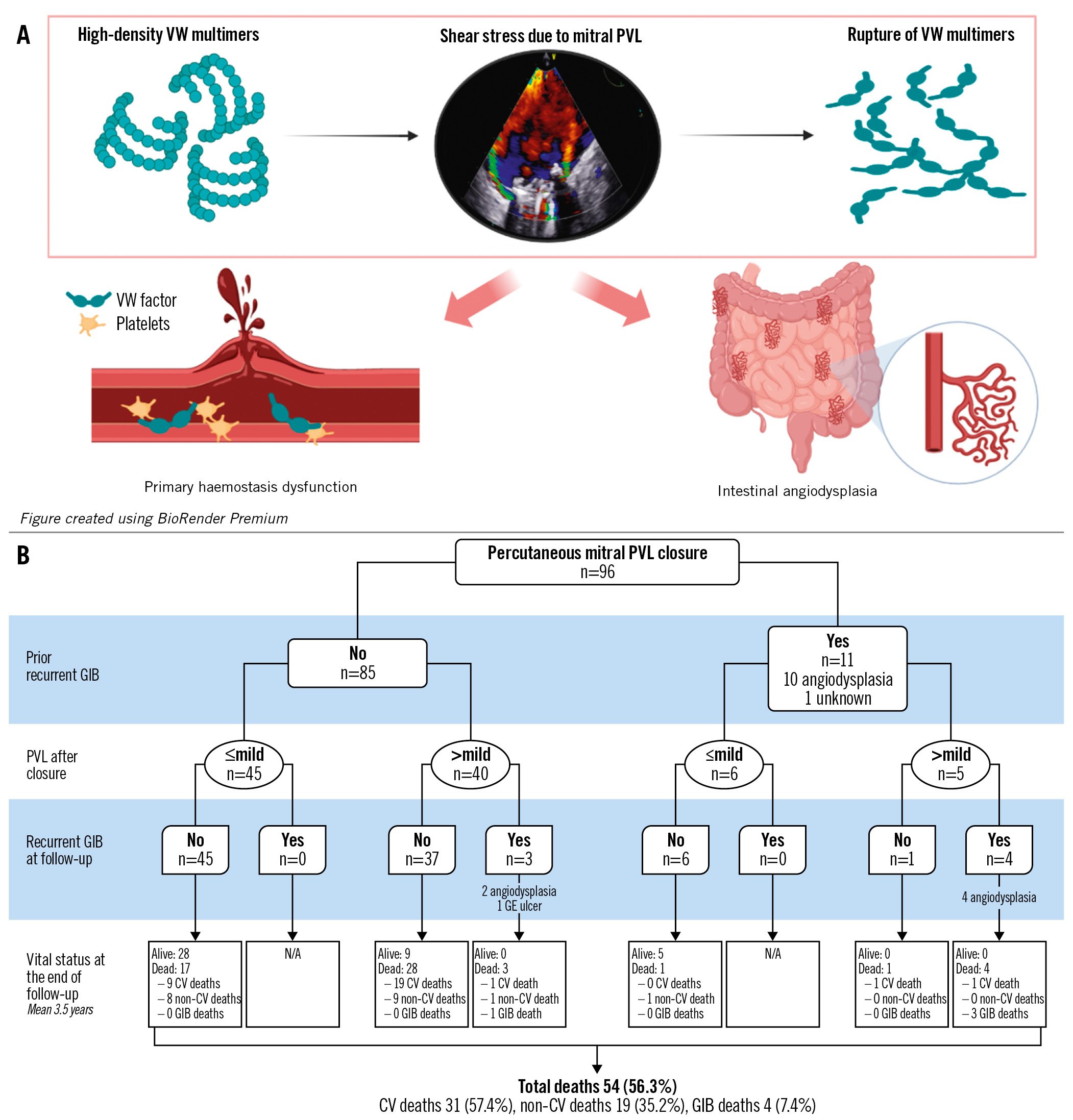
Figure 1. Link between mitral paravalvular leak and gastrointestinal bleeding. A) Potential mechanism linking the presence of mitral paravalvular leak (PVL) and gastrointestinal bleeding (GIB). B) Flowchart of GIB recurrence after mitral percutaneous PVL closure. CV: cardiovascular; GE: gastro-oesophageal; VW: von Willebrand
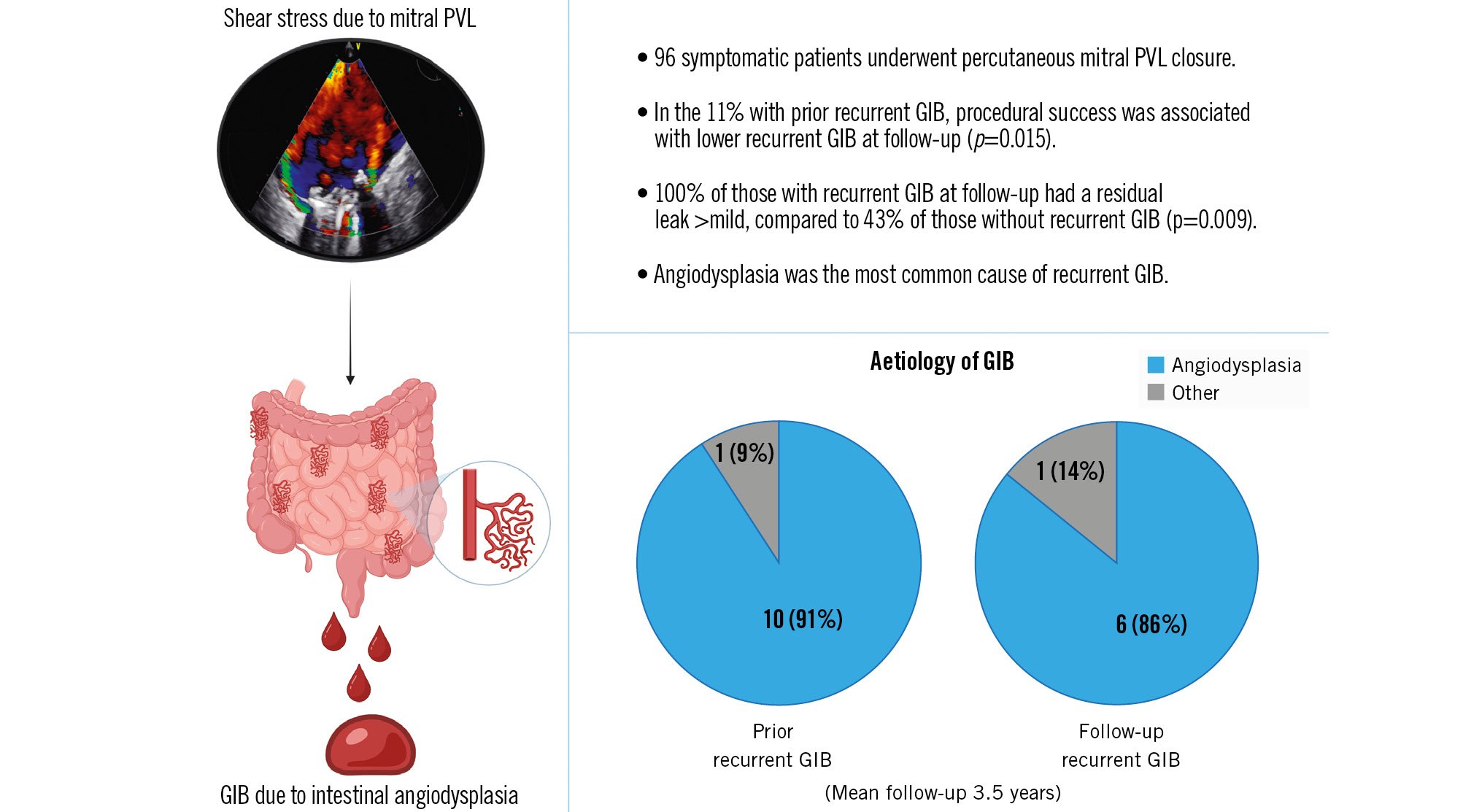
Central illustration. Recurrence of gastrointestinal bleeding after paravalvular leak closure GIB: gastrointestinal bleeding; PVL: paravalvular leak
Funding
Yassin Belahnech is supported by an unconditional research grant (Rio Hortega [CM22/00242] Instituto de Salud Carlos III and Ajut_AulaVH/2021-01).
Conflict of interest statement
The authors have no conflicts of interest relevant to the contents of this paper to declare.

