
Abstract
Aims: The aim of this study was to evaluate the impact of a horizontal aorta (HA) on device success and short-term clinical outcomes of transcatheter aortic valve implantation (TAVI).
Methods and results: We retrospectively assessed 547 consecutive patients treated with transfemoral second-generation non-balloon-expandable (NBE) (n=447) and balloon-expandable (BE) (n=100) TAVI for symptomatic severe aortic stenosis. Aortic angulation (AA) was evaluated with preprocedural computed tomography. Patients were dichotomised according to a previously established AA cut-point: HA group (AA ≥48°, n=230) and normal aorta (NA) group (AA <48°, n=317). Endpoints were considered according to the Valve Academic Research Consortium-2 definitions. Fluoroscopy time (32.8±16.4 vs 30.3±13.9 minutes, p=0.060) and radiation dose (kerma area product 120.8±99.7 vs 103.7±81.1 Gy·cm2, p=0.033) were higher in the HA group as compared to the NA group. No difference in device success was observed between patients with and without an HA (88.3% vs 88.0%, p=0.929). No differences in device success and 30-day outcomes were observed when comparing HA and NA patients, according to BE and NBE prostheses.
Conclusions: The presence of an HA has no impact on device success and short-term clinical outcomes of TAVI with either second-generation NBE or BE devices.
Introduction
Transcatheter aortic valve implantation (TAVI) has become a safe and effective treatment for patients with symptomatic severe aortic stenosis (AS) thanks to significant improvements in terms of appropriate patient selection, preprocedural planning and device design.
The diffusion of preprocedural cardiac computed tomography (CT) scans in TAVI interventions has led to recognition of “horizontal aorta” (HA) as an anatomical feature potentially related to valve positioning failure and an increased unsuccessful procedure rate1,2,3,4. A ≥48° angle between the horizontal plane and the plane of the aortic annulus in the coronal projection (aortic angulation [AA]) was previously used to define patients with HA1.
The association between increased AA (evaluated angiographically) and post-procedural paravalvular regurgitation (PVR) was first reported in 2010 among 50 patients who underwent self-expanding (SE) TAVI5. A subsequent retrospective study including 582 patients undergoing TAVI with first-generation prostheses (283 patients with HA as defined by an AA ≥48° at cardiac CT scan) reported a lower procedural success rate and higher device embolisation in HA patients with SE but not balloon-expandable (BE) valves1. Conversely, in a large retrospective registry including 3,578 patients (the CoreValve US registry), the aorto-ventricular angulation did not affect either the procedural success or clinical outcomes of TAVI in patients treated with the first-generation SE CoreValve® (Medtronic, Minneapolis, MN, USA)6.
Whether the association between HA and procedural outcomes also applies to newer-generation devices is unknown. No study to date has evaluated the performance of second-generation aortic valves in patients with HA. The new design, improved delivery systems and partial or full retrievability and repositionability of these technologies may be particularly advantageous in this setting. The aim of this study was to evaluate the impact of HA upon procedural success and short-term clinical outcomes in patients undergoing TAVI with second-generation BE and NBE valves.
Methods
STUDY POPULATION
This was a retrospective study including consecutive patients with severe symptomatic AS, who underwent transfemoral TAVI at San Raffaele Hospital (Milan, Italy) between March 2012 and December 2017 with second-generation aortic bioprostheses. Baseline clinical, echocardiographic and procedural data were recorded for all patients. All patients underwent a clinical and echocardiographic follow-up evaluation at 30 days.
Patients with a bicuspid aortic valve, primary aortic regurgitation, subaortic obstructive membrane, previous cardiac surgery (i.e., Ross intervention), those undergoing a valve-in-valve (ViV) procedure or non-transfemoral access or with failure of femoral access and those without good preprocedural CT scan were excluded.
Aortic angulation was evaluated on preprocedural CT scan for each patient. Aortic valve function was assessed by transthoracic echocardiography (TTE). The presence of concomitant coronary artery disease (CAD) was evaluated by using the preprocedural coronary CT scan and/or coronary angiography. All patients were discussed by the institutional “Heart Team”, composed of at least one interventional cardiologist, one cardiac surgeon, one cardiac imaging specialist and one anaesthesiologist.
CT SCAN
The electrocardiographically gated preprocedural CT scan was analysed by an expert radiologist. AA was defined as the angle between the horizontal plane and the plane of the aortic annulus, calculated from a coronal projection at the level of the aortic annulus (Figure 1). An AA ≥48° was previously shown to predict lower device success in SE TAVI best1 and was then used to define HA. The radiologist who retrospectively evaluated AA on the preprocedural CT scan was unaware of the implanted valve type.
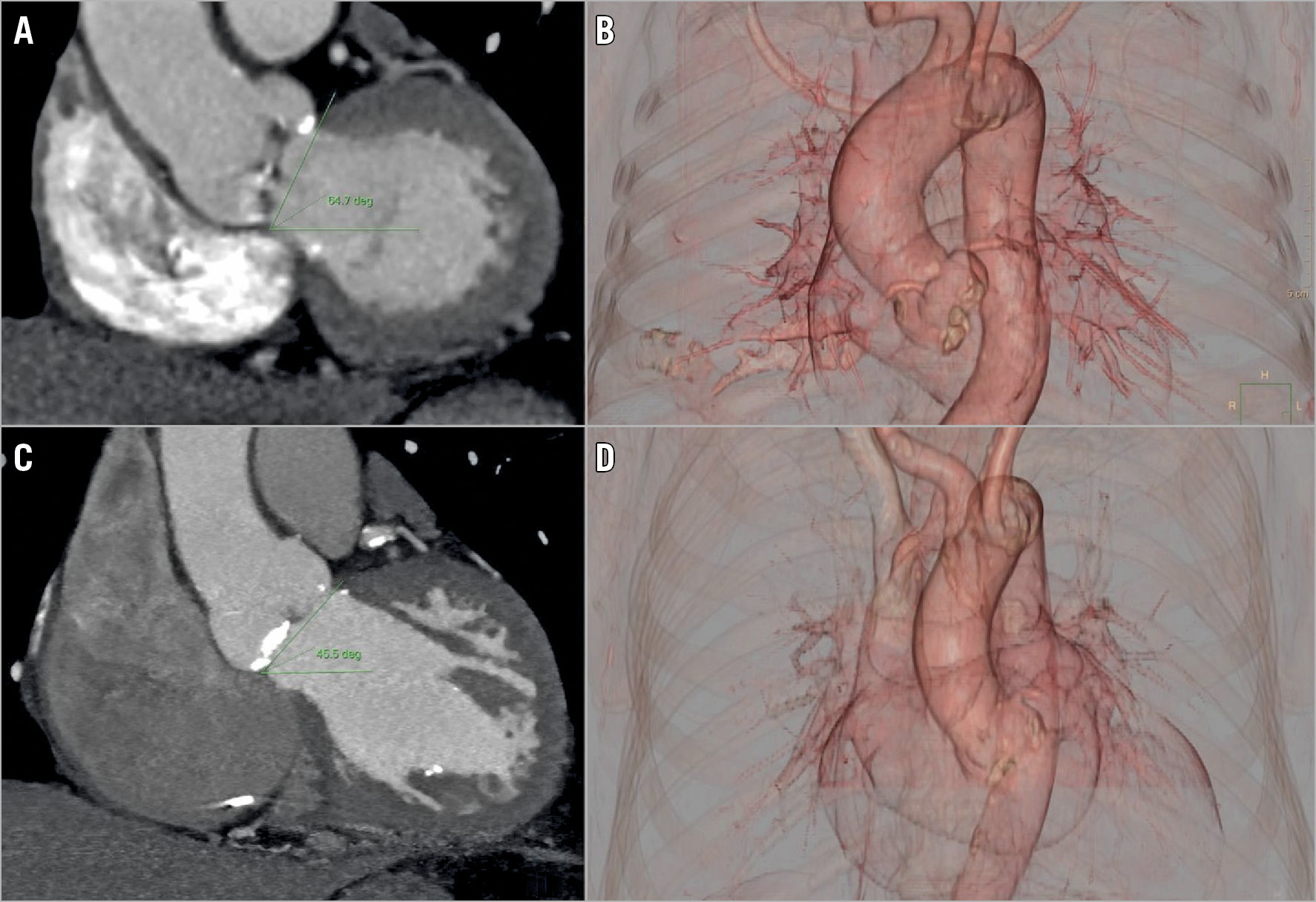
Figure 1. Computed tomography evaluation of aortic angulation. Measurement of aortic angulation (AA) is performed in a coronal projection of computed tomography angiography (left panels, A & C). AA is defined as the angle between the horizontal plane and the plane of the aortic annulus. 3D volume rendering reconstruction (right panels, B & D ). Two cases of normal aorta with AA=45.5° (upper panels, A & B) and horizontal aorta with AA=64.7° (bottom panels, C & D) are presented.
TAVI DEVICE
The SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA), CoreValve Evolut™ R and PRO (Medtronic), ACURATE neo™ (Boston Scientific, Marlborough, MA, USA), Portico™ (St. Jude Medical/Abbott Vascular, St. Paul, MN, USA), Lotus™ (Boston Scientific) and Direct Flow (Direct Flow Medical, Santa Rosa, CA, USA) were the prostheses utilised in the study population. Prosthetic valve type/size and vascular accesses were left to the discretion and preference of the operator.
OUTCOME ANALYSIS
The primary endpoints of the study were device success, post-procedural aortic regurgitation (AR) degree, and in-hospital and 30-day mortality. Device success was defined according to Valve Academic Research Consortium-2 (VARC-2) definitions7.
Secondary endpoints were procedural and in-hospital complications including valve embolisation, need of a second valve, aortic rupture or dissection, myocardial infarction (MI), cardiac tamponade, need of emergency surgery, stroke or transient ischaemic attack (TIA), major vascular complications, permanent pacemaker (PM) implantation, acute kidney injury (AKI), sepsis, and adverse events during the follow-up including hospitalisation for cardiovascular causes, heart failure, endocarditis, valve thrombosis, stroke or TIA, MI, angina and AR at 30 days.
All TAVI endpoints were recorded according to the VARC-2 criteria7.
Acute procedural success and short-term clinical outcomes were compared between patients with an AA <48° versus those with an AA ≥48° (a) in the overall population and (b) according to the implanted valve type (BE/NBE). The association between AA as a continuous variable and device success was further evaluated.
STATISTICAL ANALYSIS
Variables are presented as mean±SD for continuous variables and as number (percentage) of patients in each group for categorical variables. The Student’s t-test and the Pearson’s chi-square test or Fisher’s exact test were used to evaluate statistical significance between continuous and categorical variables, as appropriate. All variables were normally distributed according to the Kolmogorov-Smirnov test. The ANOVA test was utilised to evaluate the association between the continuous variables and categorical variables. Multivariate analysis was performed to evaluate the influence of other independent variables on device success.
Results
BASELINE CHARACTERISTICS
Between March 2012 and December 2017, 706 TAVI procedures with second-generation devices were performed in our centre. Thirty-seven patients were treated with TAVI for surgical bioprosthesis failure, 58 patients underwent TAVI using a non-transfemoral access, 12 patients had pure AR, 25 had a bicuspid valve, 23 patients did not undergo preprocedural CT scan, 2 cases had femoral access failure, 1 case was carried out in a previous Ross intervention and 1 in a subaortic obstructive membrane, and were therefore excluded. Five hundred and forty-seven patients were treated with TAVI by transfemoral access using the SAPIEN 3 (n=100; 18%), CoreValve Evolut R (n=136; 25%), CoreValve Evolut PRO (n=12; 2%), ACURATE neo (n=46; 8%), Portico (n=50; 9%), Lotus (n=85; 16%) and Direct Flow (n=118; 22%), and were thus included. A detailed study flow chart is provided in Figure 2. Mean AA was 46.1±10°; 317 patients had an AA <48° (AA=39.3±6.3°), and 230 had an AA ≥48° (AA=55.3±5.8°). No significant differences in the type of valve and in NBE versus BE valves were observed between patients with and without HA. The prostheses chosen for implantation in HA and NA patients are summarised in Figure 2.
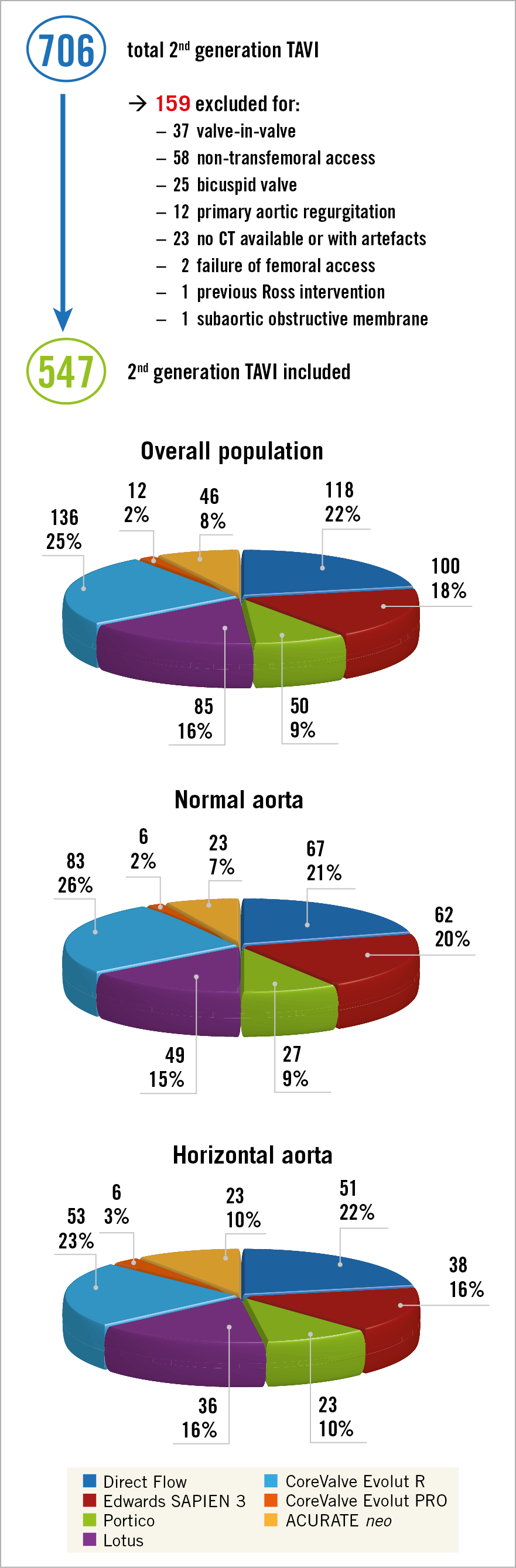
Figure 2. Screening process and types of valve in overall, NA and HA populations. CT: computed tomography; HA: horizontal aorta; NA: normal aorta; TAVI: transcatheter aortic valve implantation
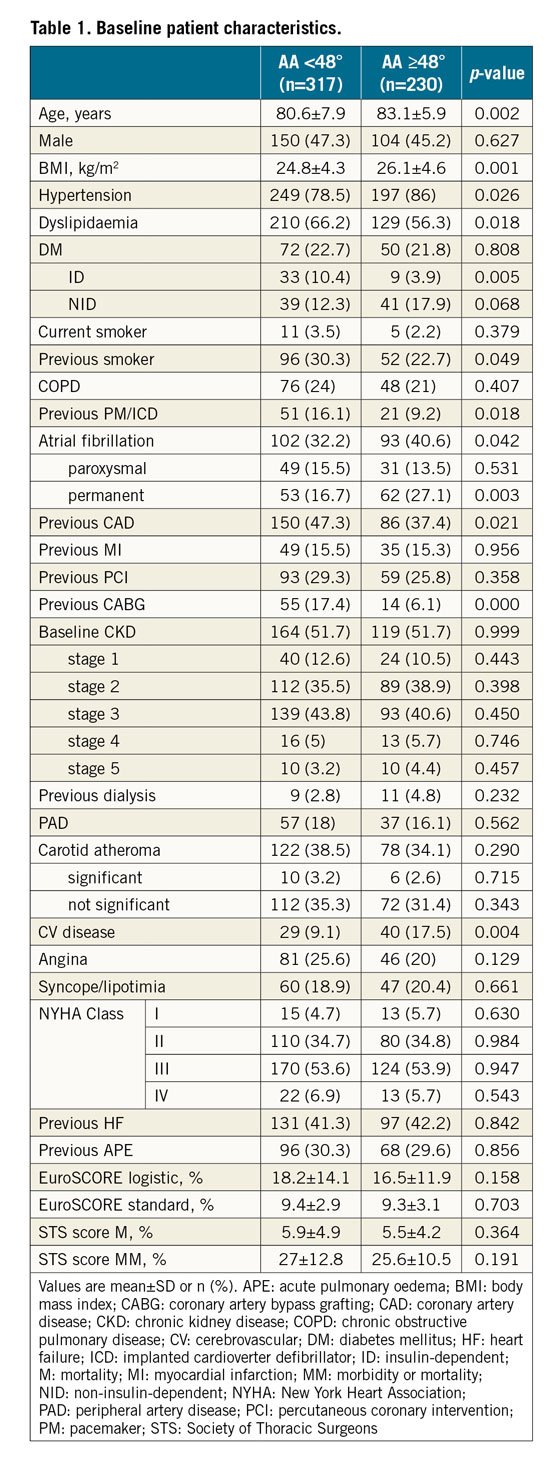
The baseline clinical characteristics of the study population are presented in Table 1. HA was more common in older patients (83.1±5.9 vs 80.6±7.9 years; p=0.002) and was associated with higher body mass index (BMI) (26.1±4.6 vs 24.8±4.3 kg/m2; p=0.001), hypertension (86.0% vs 78.5%; p=0.026), atrial fibrillation (40.6% vs 32.3%; p=0.042) and cerebrovascular disease (17.5% vs 9.2%; p=0.004). On the other hand, ex-smoker status (22.7% vs 30.3%; p=0.049), previous PM or implantable cardioverter defibrillator (ICD) (9.2% vs 16.1%; p=0.018), CAD (37.4% vs 47.3%; p=0.021) and coronary artery bypass grafting (CABG) (6.1% vs 17.4%; p<0.001) were more frequent in the NA group. All other baseline characteristics were similar between the groups (Table 1).
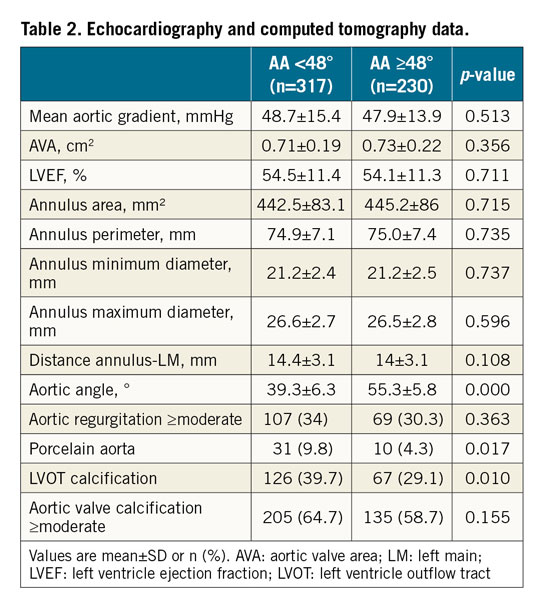
The preprocedural echocardiographic and CT data of the study population are presented in Table 2. Patients with HA less frequently had porcelain aorta (4.3% vs 9.8%; p=0.017) and left ventricle outflow tract (LVOT) calcification (29.1% vs 39.7%; p=0.010). All other imaging variables were similar between the groups.
HA VERSUS NA OUTCOMES
Procedural details, clinical outcomes, and 30-day follow-up for the HA and NA groups are reported in Table 3 and Figure 3. Higher radiation dose (kerma area product 120.8±99.7 vs 103.7±81.1 Gy·cm2; p=0.033) and a trend towards higher fluoroscopy time (32.8±16.4 vs 30.3±13 minutes; p=0.060) were observed in the HA group. Device success was similar between groups (88.3% vs 88.0%; p=0.929). There was a higher rate of AR ≥moderate at one-month follow-up in the NA group (21.7% vs 30.7%; p=0.045). All other procedural, clinical and follow-up data were similar between the two groups (Table 3). There was no significant association between the aortic angle as a continuous variable and the device success (p=0.614). The neutral impact of AA on device success was maintained also after adjustment for relevant baseline characteristics, LVOT calcification, moderate to severe aortic calcification and post-dilation (Supplementary Table 1). Also, removing unavailable valves (Lotus and Direct Flow) from the analysis, device success remains the same, without significant differences between NA and HA (84.1% vs 86.7%; p=0.498).
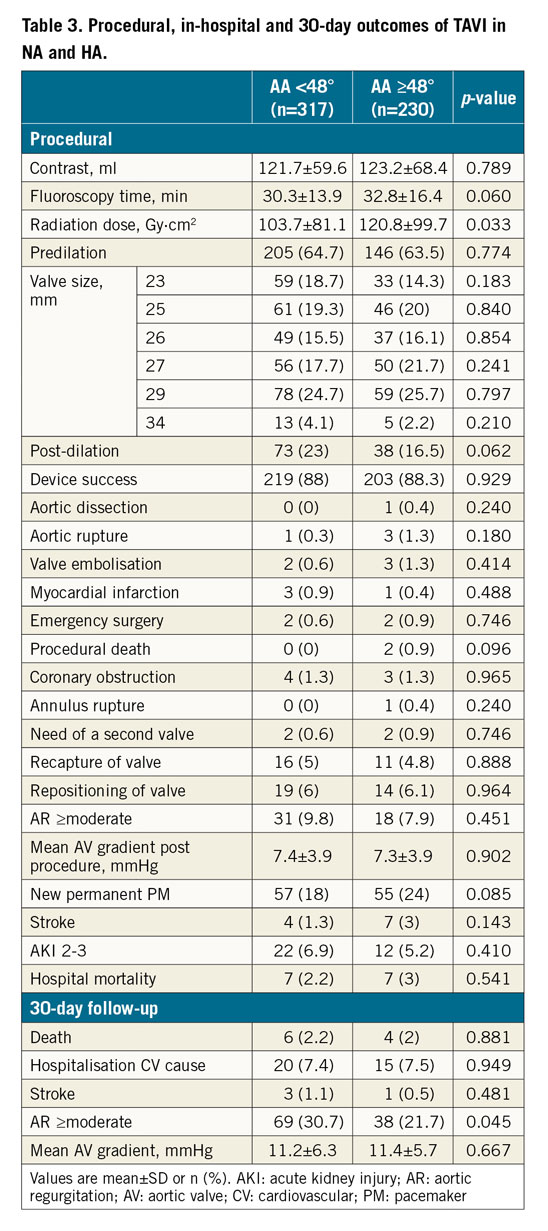
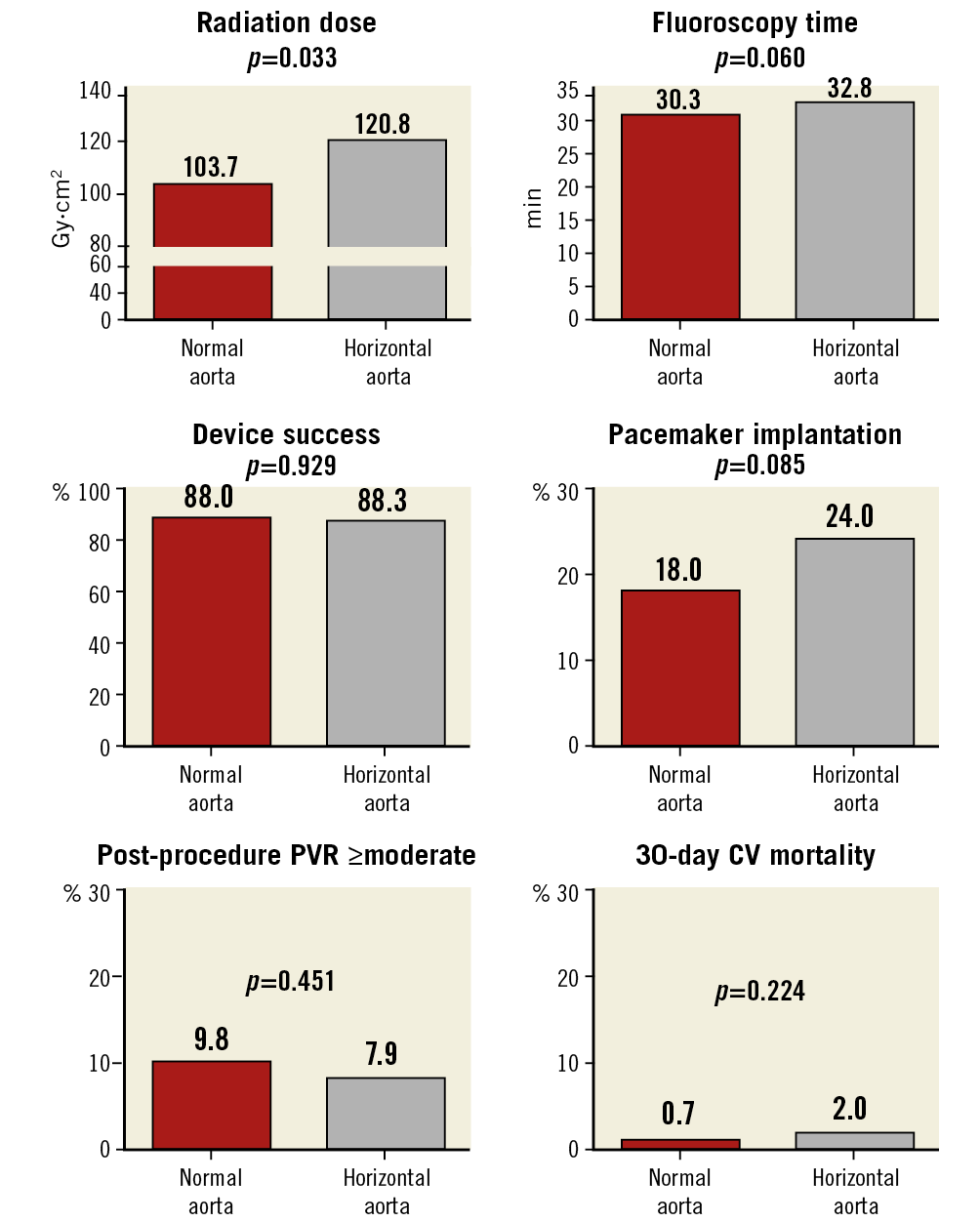
Figure 3. HA versus NA at procedure, discharge, and 30-day follow-up. Fluoroscopy time is expressed in minutes, radiation dose in Gy·cm2, device success, post-procedure PVR, pacemaker implantation and 30-day CV mortality in percentage (%). CV: cardiovascular; HA: horizontal aorta; NA: normal aorta; PVR: paravalvular regurgitation
HA VERSUS NA OUTCOMES ACCORDING TO IMPLANTED VALVE: BE/NBE
Procedural details, clinical outcomes, and 30-day follow-up stratified by HA status for patients who underwent TAVI with BE or NBE valves are presented in Table 4 and Figure 4. TAVI was performed by using BE and NBE valves in 100 (18.3%; n=38 HA, n=62 NA) and 447 (81.7%; n=192 HA, n=255 NA) patients, respectively. Radiation dose and fluoroscopy time were numerically higher for HA procedures in both NBE and BE groups. Among patients treated with NBE valves, a significantly higher post-dilatation rate was observed in NA patients (17.7% vs 26.0%; p=0.040). There were no significant differences in device success rate, procedural and in-hospital complications, post-procedural AR, and 30-day adverse events including mortality between the HA and NA groups both in patients treated with BE and in those treated with NBE valves. Among patients treated with BE valves, a significantly higher rate of permanent PM implantation was observed in HA patients (27.0% vs 8.1%; p=0.011). Among patients treated with NBE valves, a trend towards higher ≥moderate AR at one month was observed in NA patients (22.1% vs 31.5%; p=0.056). All other procedural, clinical and follow-up data were similar between the two groups (Table 4).
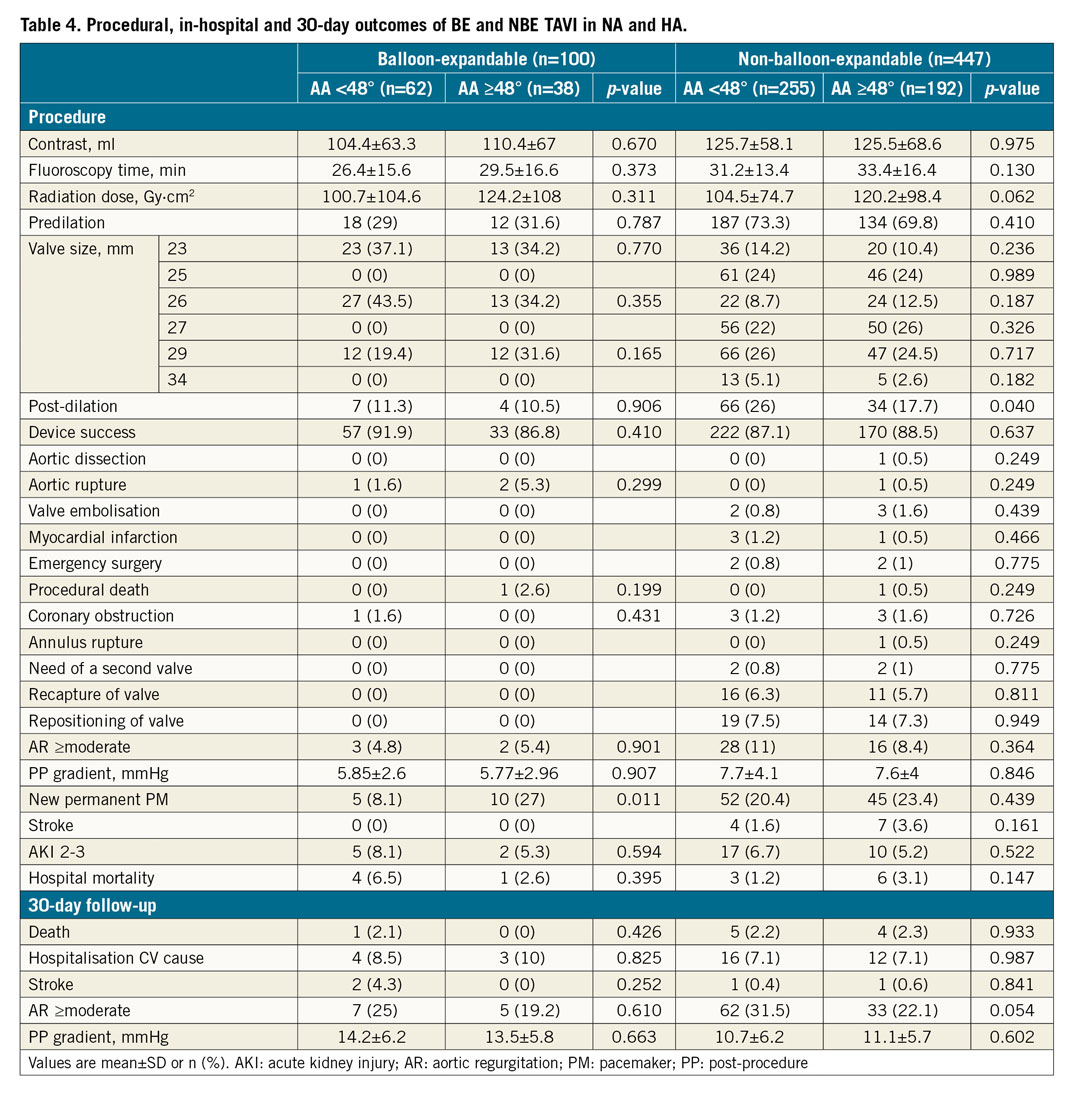
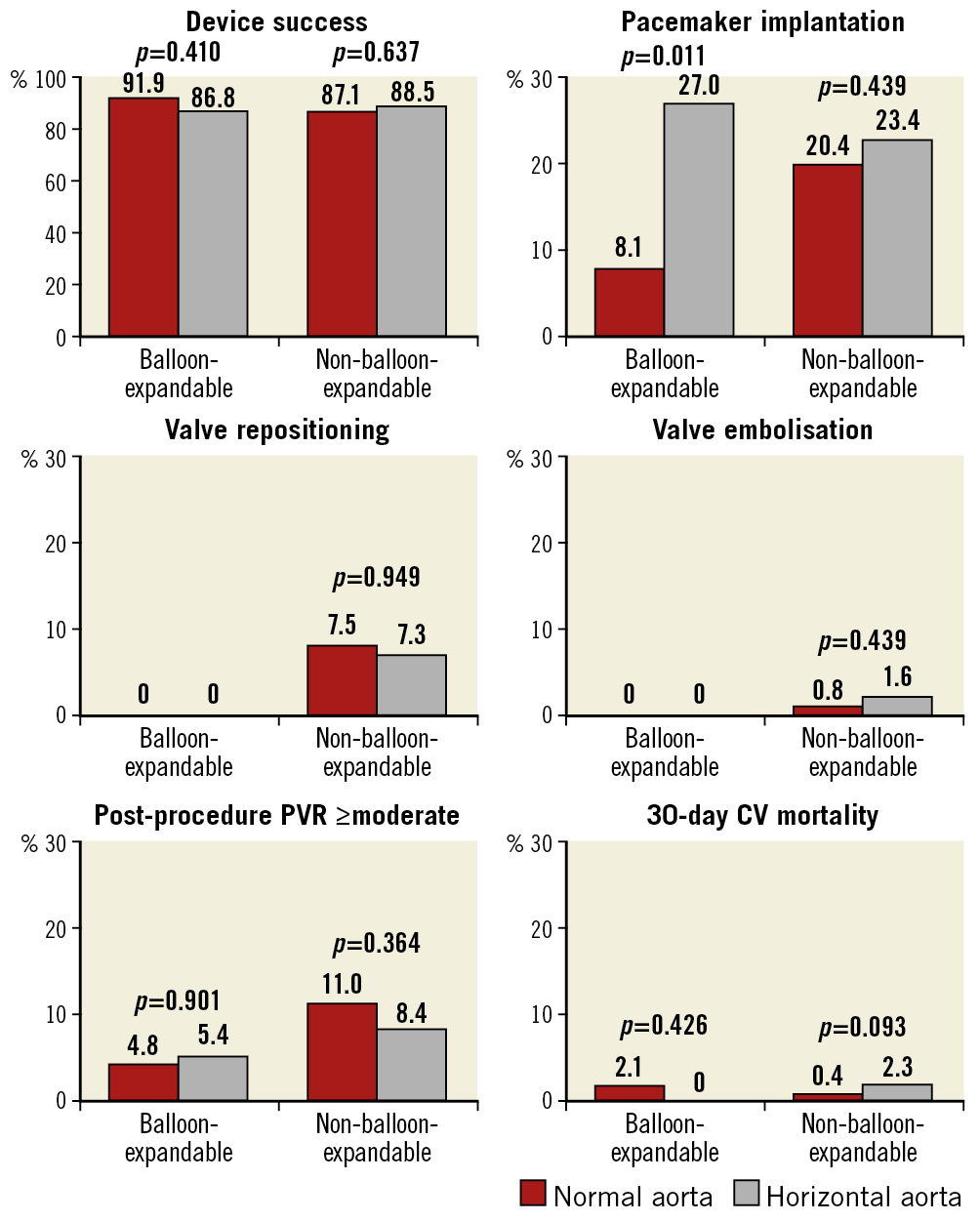
Figure 4. Outcomes of BE versus NBE valves in NA compared to HA at procedure, discharge, and 30-day follow-up. All values are expressed in percentage (%). BE: balloon-expandable; CV: cardiovascular; HA: horizontal aorta; NA: normal aorta; NBE: non-balloon-expandable; PVR: paravalvular regurgitation
Discussion
The main findings of this study are:
1) Second-generation TAVI devices have a similar success rate (approximately 88%) in patients with and without HA.
2) Procedural success and short-term outcomes do not seem to be influenced by aortic angulation for both NBE and BE second-generation devices.
3) TAVI procedures in HA patients are associated with a higher radiation dose and a tendency to a higher fluoroscopy time.
Patients with HA have been identified as a subgroup at increased risk of complications and adverse clinical outcomes with first-generation SE TAVI1,2,3,4. This may be due to differences in valve characteristics. The correlation between higher AA and increased post-procedural AR was first observed by Sherif et al in 20105 based on a small group of 50 patients treated with the first-generation SE CoreValve with aorto-ventricular angulation assessed by left ventriculography in a right anterior oblique projection of 30° during preparation of the patient for TAVI.
With the advent of the CT scan as the preprocedural “gold standard”5, AA has become more reliably assessed and new evidence added to this field. In 2016, Abramowitz et al showed, in a group of 582 patients treated with first-generation valves, that an increased AA adversely influenced acute procedural success following TAVI with the use of SE valves but not with the use of BE valves. In that study, SE valves were associated with a higher rate of post-dilatation, need of a second valve, valve embolisation, post-procedural PVR and fluoroscopy time in patients with HA as compared to NA patients (although with no impact on mortality at 30-day and six-month follow-up)1. This finding was not confirmed in a large cohort (n=3,578) of patients undergoing TAVI with the CoreValve device reported by Popma et al where the degree of AA did not have any impact upon procedural success or clinical outcomes6.
While previous reports were limited to first-generation devices, our study demonstrates that, in second-generation valves, the presence of HA seems not to have an impact on outcomes regardless of the type of valve implanted. In this study the rate of the primary endpoint was similar between NA and HA (88% vs 88.3%; p=0.929), also when stratified by valve type (BE: 91.9% vs 86.8%; p=0.410; NBE: 87.1% vs 88.5%; p=0.637). The reason for the consistently successful implantation of the newer NBE devices across a range of AA may lie in their structural improvement. In particular, the repositionability of new NBE valves offers the possibility of optimising implantation depth; the improved design of the stent frame and the skirt may reduce the risk of AR and of coronary obstruction; the stabilisation arches and the new delivery systems allow a better and more effective coaxial alignment to the annulus. These features may be of particular benefit in HA anatomies, translating into similar device success and similar short-term outcomes compared to BE devices. Importantly, post-procedure valve haemodynamic function and clinical outcomes at discharge and at 30-day follow-up were similar between patients with and without HA, also when considering the use of NBE or BE valves.
The higher rate of new permanent PM implantation in patients with AA ≥48° undergoing TAVI with BE valves is difficult to explain.
The higher radiation dose and numerically higher fluoroscopy time observed in our cohort are consistent with the concept of more technically demanding procedures in angulated aortas, regardless of whether the implanted device is BE or NBE. However, the similar rate of procedural complications between patients with and without HA observed in our study supports the idea that such procedural challenges do not translate into worse outcomes in HA anatomies, possibly due to technological improvements.
In conclusion, TAVI in HA seems to be safe and feasible with both BE and NBE valves. This finding suggests that other structural characteristics should be taken into consideration when tailoring valve choice to a specific patient.
Limitations
This was a single-centre retrospective study with short follow-up. Second, only about one in five implanted valves was BE. However, previous concerns regarding the safety of TAVI in HA were limited to NBE valves, which were well represented in our analysis. This recognition, together with the absence of numerical differences in outcome rates between NA and HA patients receiving a BE valve, supports the favourable clinical profile in HA anatomies of both second-generation device types.
Conclusions
The use of contemporary second-generation NBE or BE TAVI prostheses in patients with HA is associated with similar and acceptable rates of device success and short-term clinical outcomes comparable to those of patients without HA.
|
Impact on daily practice A few studies have shown conflicting data about the impact of a horizontal aorta on clinical and procedural outcomes in patients undergoing TAVI, and only with first-generation valves. Our study showed that a horizontal aorta has no impact on device success and short-term clinical outcomes of TAVI with either second-generation self-expanding or balloon-expandable prostheses. This might alter the way we choose the appropriate prosthesis type in patients with horizontal aorta. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

