Abstract
Aims: This study aimed to investigate the impact of end-stage renal disease (ESRD) on clinical outcomes following infrapopliteal intervention in critical limb ischaemia patients with tissue loss.
Methods and results: This retrospective single-centre study enrolled 92 consecutive patients (117 limbs) undergoing infrapopliteal intervention for the treatment of ischaemic tissue loss. The primary outcomes were the wound healing rate, the clinically driven reintervention rate and the limb salvage rate. The secondary outcome was amputation-free survival. The pedal arch was significantly (p=0.002) more diseased in ESRD patients than in non-ESRD patients. ESRD patients demonstrated a significantly lower wound healing rate (hazard ratio [HR], 0.552; 95% CI: 0.319-0.957; p=0.034) and a higher reintervention rate (HR, 1.988; 95% CI: 1.135-3.482; p=0.016). However, there was no significant difference in limb salvage rate between patients with and without ESRD. Age (HR, 1.056; 95% CI: 1.020-1.094; p=0.002), ESRD (HR, 2.239; 95% CI: 1.138-4.407; p=0.020), heart failure (HR, 2.360; 95% CI: 1.295-4.302; p=0.005) and infectious wound (HR, 2.017; 95% CI: 1.145-3.552; p=0.015) were independently associated with death or major amputation.
Conclusions: ESRD patients yielded a more affected pedal arch and were at approximately twice the risk of wound healing failure, need for reintervention, and death or major amputation compared to non-ESRD patients.
Introduction
The growing global burden of end-stage renal disease (ESRD) and peripheral artery disease (PAD) has been recognised as a significant public health issue worldwide1,2. ESRD can accelerate the deterioration of PAD and provoke critical limb ischaemia (CLI) with extensive vessel calcification and impaired vascular resistance3-5. Since infrapopliteal artery disease is a key feature of CLI, whether single- or multi-segment6, bypass surgery has served as the exclusive remedy even in ESRD patients. However, the surgical approach poses specific technical and clinical challenges due to the high underlying cardiovascular risk and inevitable vessel complexity present in cases of ESRD patients7-10.
Catheter-based infrapopliteal intervention is gaining popularity as the treatment of CLI. Also, there is a consensus that the prognosis of CLI patients with ESRD is dismal compared to those without ESRD even after endovascular intervention11,12. However, the performance of infrapopliteal intervention in ESRD patients has not yet been fully elucidated in real-world practice. Thus, the aim of this study was to clarify infrapopliteal lesion characteristics and investigate the clinical outcomes of infrapopliteal intervention in CLI patients with ESRD who are on haemodialysis.
Methods
POPULATION
One hundred and fifty-seven consecutive patients (200 limbs) with ischaemic tissue loss were referred to our hospital (Kishiwada Tokushukai Hospital) between 2002 and 2009, as described elsewhere11. Seven limbs in six patients were treated primarily with bypass surgery or major amputation, and 193 limbs in 151 patients primarily received endovascular intervention. Infrapopliteal revascularisation was subsequently required on 117 limbs in 92 patients based on clinical manifestations and microcirculation examinations. All subjects were amenable to endovascular treatment because of lesion involvement, run-off conditions, and/or clinical comorbidities, including ESRD on chronic haemodialysis. An institutional review committee approved this retrospective study. Analysed patient data were derived from medical records and telephone contact (10 limbs), and follow-up was concluded in April 2011 (6 limbs were lost to follow-up).
INTERVENTIONAL PROCEDURE
Our interventional procedures have been described elsewhere11,13. In the treatment of chronic total occlusions, a variety of endovascular crossing techniques was considered based on operator discretion14. Stent-assisted angioplasty using coronary bare metal stents was our endovascular strategy for the establishment of at least one straight-line flow to the foot. The treatment vessel was selected based on angiographic interpretation whereby the occluded vessel with evidence of reconstitution was indicated for endovascular recanalisation. Coronary bare metal stents were implanted in cases of failed balloon angioplasty due to significant recoil, flow-limiting dissection, abrupt closure, or repeat early reocclusion. In addition, below-the-ankle lesions were also treated if deemed necessary. Procedural success was defined as recanalisation of at least one straight-line flow to the pedal arch with <30% residual stenosis after balloon angioplasty with bail-out stenting. A bolus of 3,500-5,000 IU heparin was administered through the arterial sheath with additional heparin given intravenously during the procedure to maintain the activated clotting time at >200 sec. Dual antiplatelet therapy was administered to all patients from at least two days before the procedure to 30 days after, and patients were then prescribed lifelong aspirin unless contraindicated or discontinued due to side effects.
POST-INTERVENTION FOLLOW-UP
For close follow-up of clinical symptoms, all cases were scheduled to undergo serial (before, next day, one week, at one, three, six months, and every six months) examinations with duplex ultrasonography and checking of the dorsalis pedis and/or paramalleolar posterior tibial artery pulses to determine the need for reintervention. Due to its limitations caused by the presence of calcified vessels, duplex ultrasonography has only served as an adjunctive tool for non-invasive assessment of infrapopliteal artery patency. Since 2005, our decision-making process regarding revascularisation has involved the serial evaluation of skin perfusion pressure (SPP). A SensiLase™ PAD 3000 device (Väsamed, Eden Prairie, MN, USA) was used to assess SPP, as described in a previous report6. An SPP <40 mmHg on either the dorsal or plantar side was a definite indication for revascularisation because of the low likelihood of wound healing15. Acute occlusion, subacute occlusion, and haemodynamically significant restenosis were diagnosed by confirmatory angiography and were treated with reintervention until complete wound healing was achieved or for relief of recurrent ischaemic symptoms. Reintervention was performed using balloon angioplasty with bail-out stenting. Wounds were managed by specialised physicians and nurses during hospitalisation and on an outpatient basis. Debridement or minor amputation was performed for further removal of residual infectious or necrotic tissue.
CLASSIFICATION OF INFRAPOPLITEAL SEGMENTS
Figure 1 shows the classification adopted in this study to divide proximal and distal segments in the crural artery above the ankle. The pedal arch below the ankle was classified as type 1, 2, or 3 based on the published literature11.
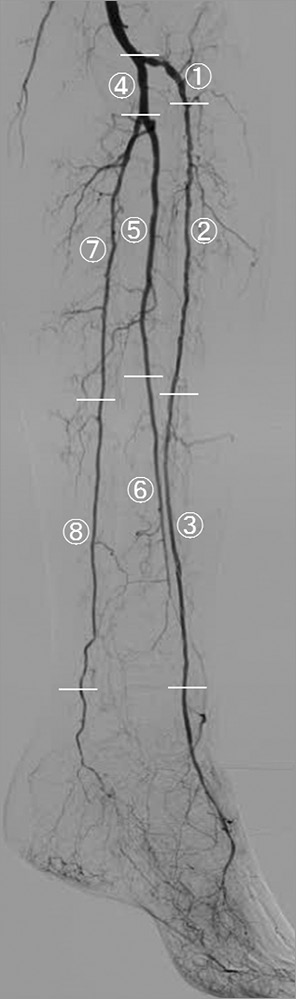
Figure 1. Classification of crural artery segments. The anterior tibial artery is divided into three segments: AT1 comprises the ostium to the first bend, and the remaining segment is divided equally in two, resulting in AT2 and AT3. The tibioperoneal trunk is divided into the peroneal and posterior tibial arteries. The peroneal artery is divided into two equal segments, Pero1 and Pero2. The posterior tibial artery is divided into two equal segments, PT1 and PT2. ![]() AT1;
AT1; ![]() AT2;
AT2; ![]() AT3;
AT3; ![]() Tibioperoneal trunk;
Tibioperoneal trunk; ![]() Pero1;
Pero1; ![]() Pero2;
Pero2; ![]() PT1;
PT1; ![]() PT2
PT2
Statistical analysis
The primary outcomes were wound healing rate (indicating rate of complete wound healing), clinically driven reintervention rate and limb salvage rate, and the secondary outcome was amputation-free survival (AFS). Categorical variables were analysed using either Pearson’s chi-squared test or Fisher’s exact test. Continuous variables were reported as means±SD and compared using an unpaired t-test. The Kaplan-Meier method was employed for the analysis of wound healing, reintervention, limb salvage and AFS. Limb salvage was defined as freedom from major amputation (above the ankle). A backward stepwise regression analysis was performed with the Cox proportional hazards regression model to adjust for confounding factors and to identify independent outcome predictors. Covariates included in the model were age, dyslipidaemia, diabetes mellitus, ESRD, stroke, heart failure, infectious wounds, and final pedal arch classification. P-values <0.05 were considered significant. Statistical analysis was performed with SPSS Version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
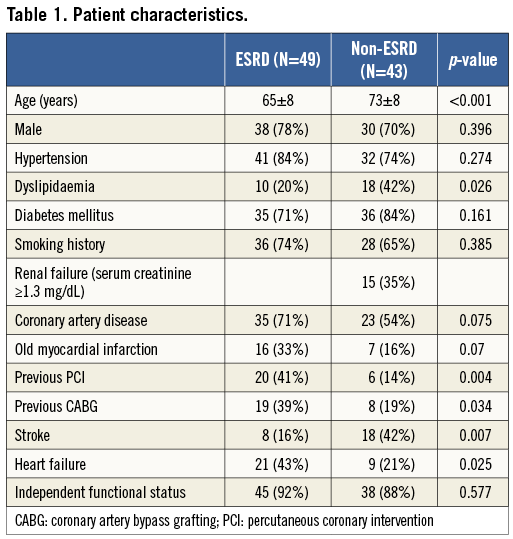
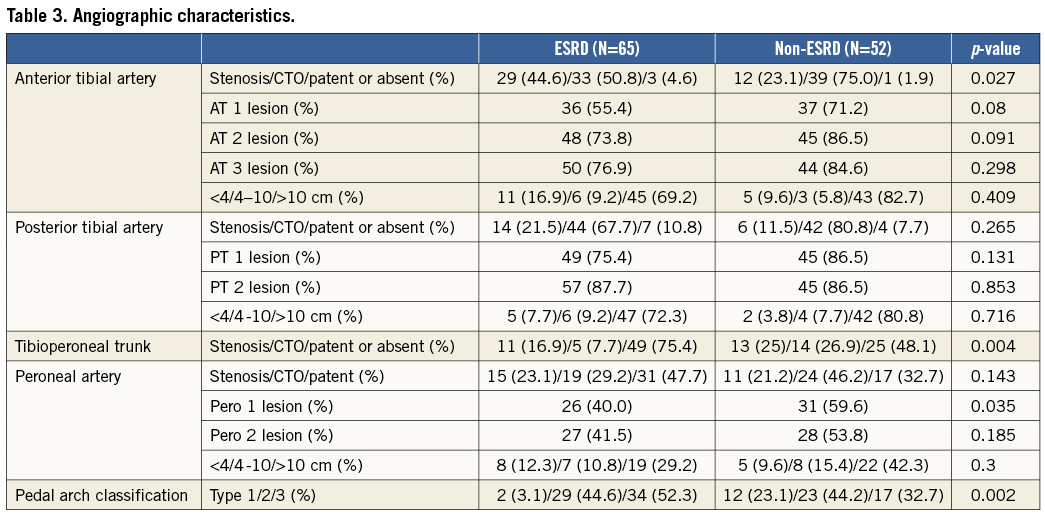
The patient and limb characteristics of 117 limbs in 92 patients are shown in Table 1 and Table 2. ESRD patients were younger and less hyperlipidaemic, and had a significantly greater history of coronary revascularisation and heart failure. The angiographic characteristics of the 117 limbs are shown in Table 3. Above the ankle, complicated lesions which included total occlusion were significantly more frequent in the anterior tibial artery (p=0.027) and the tibioperoneal trunk (p=0.004) in non-ESRD patients than in ESRD patients. Below the ankle, the pedal arch was significantly (p=0.002) more diseased in ESRD patients than in non-ESRD patients.
Procedural success was observed in 106 limbs in 85 patients, specifically in 87.7% (57) of ESRD patients and 94.2% (49) of non-ESRD patients (p=0.229). Thirty-day mortality was numerically higher in ESRD (3.5%) than in non-ESRD patients (0%) (p=0.498). In addition to revascularisation, the need for debridement or minor amputation was numerically more frequent in patients with ESRD than in those without (46% vs. 33%; p=0.174). The number of debridement procedures per limb, when required, was 1.5±0.6 (range: 1-3) in ESRD patients and 1.4±0.8 (range: 1-4) in non-ESRD patients (p=0.087).
The wound healing rate was significantly (p=0.028, by log-rank) lower in ESRD patients than in non-ESRD patients (Figure 2A). The Cox proportional hazards model demonstrated a significantly lower wound healing rate in ESRD than in non-ESRD patients (p=0.034) (Figure 2B), and there was a 45% decrease in hazard for wound healing for ESRD patients compared to non-ESRD patients (HR, 0.552; 95% CI: 0.319-0.957) (Table 4). In addition, the Cox model identified diabetes mellitus, the presence of infectious wound and final pedal arch type as adverse predictors of wound healing. Also, according to a post hoc Kaplan-Meier analysis, there was a significant difference (p<0.001) in wound healing among the four groups divided by the presence of diabetes mellitus and ESRD. In particular, the wound healing rate differed significantly between diabetic and non-diabetic patients, with the exception of diabetic patients without ESRD versus non-diabetic patients with ESRD (Figure 2C).
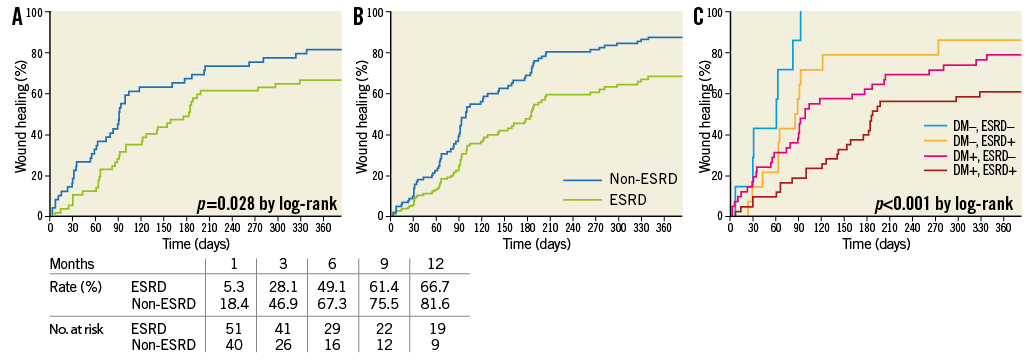
Figure 2. Wound healing rate. A) Kaplan-Meier curves. There was a significant (p=0.028) difference between the two. B) Cox adjusted analysis. There was a significantly (p=0.034) lower wound healing rate in patients with ESRD compared to those without ESRD. C) Post hoc Kaplan-Meier analysis. There was a significant difference (p<0.001) among the four groups defined by the presence or absence of diabetes and ESRD. In particular, the wound healing rate differed significantly between diabetic and non-diabetic patients except when comparing diabetic patients without ESRD and non-diabetic patients with ESRD. DM+, ESRD+ versus DM–, ESRD+; p=0.005 by log-rank. DM+, ESRD+ versus DM–, ESRD–; p<0.001 by log-rank. DM+, ESRD– versus DM–, ESRD+; p=0.397 by log-rank. DM+, ESRD– versus DM–, ESRD–; p=0.006 by log-rank. DM+, ESRD+ versus DM+, ESRD–; p=0.028 by log-rank. DM–, ESRD+ versus DM–, ESRD–; p=0.049 by log-rank.
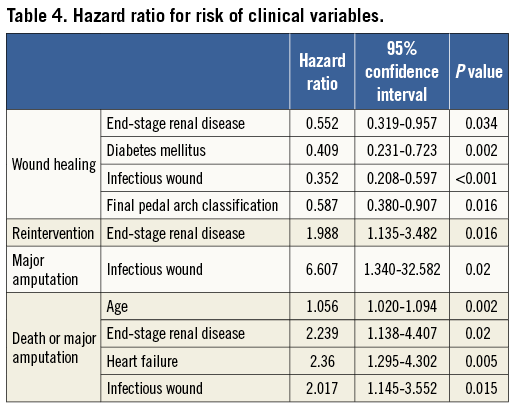
During the clinical follow-up period of 27±22 months (range 1-99), clinically driven reintervention rate was significantly higher (p=0.013, by log-rank) in ESRD patients than in non-ESRD patients (Figure 3A). According to the Cox model, ESRD patients also displayed a significantly higher (p=0.016) reintervention rate than non-ESRD patients (Figure 3B), and there was a 98% increase in hazard ratio for reintervention for ESRD patients (HR, 1.988; 95% CI: 1.135-3.482) (Table 4). According to a post hoc Kaplan-Meier analysis, there was a trend (p=0.060) towards a significant difference in reintervention rate among the four groups defined by the presence or absence of diabetes mellitus and ESRD (Figure 3C). Also, there was a trend (p=0.058) towards a higher number of reinterventions in ESRD patients compared to non-ESRD patients (1.5±1.7, range 0-9 vs. 0.9±1.5, range 0-7). Also, ESRD patients showed a trend towards frequent reintervention (61%, 35 limbs vs. 43%, 21 limbs, p=0.057). However, among reintervention cases, there was no significant difference in the number of reinterventions (ESRD, 2.4±1.6; non-ESRD, 2.0±1.6; p=0.373).
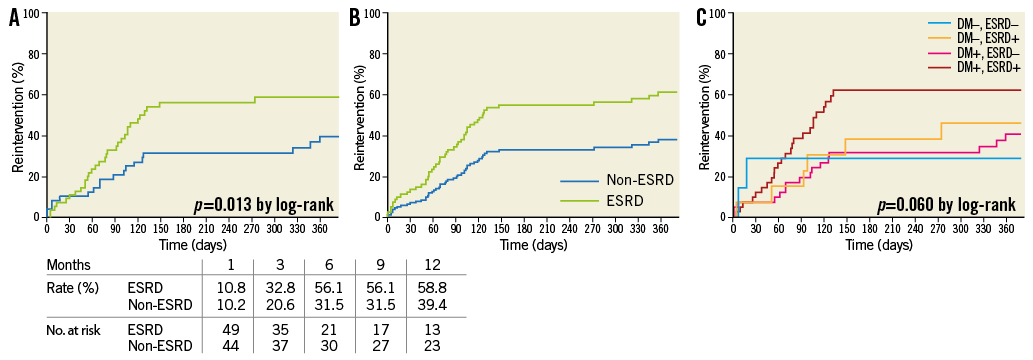
Figure 3. Clinically driven reintervention rate. A) Kaplan-Meier curves. There was a significant (p=0.013) difference between the two groups. B) Cox adjusted analysis. There was significantly (p=0.016) higher reintervention in patients with ESRD compared to those without ESRD. C) Post hoc Kaplan-Meier analysis.
Regarding limb salvage rate, there was no significant difference (p=0.757, by log-rank) between the two groups (Figure 4A). This was confirmed by the Cox model (Figure 4B), which also identified infectious wounds as an independent predictor of major amputation (Table 4). Even in a post hoc Kaplan-Meier analysis, the limb salvage rate did not differ significantly among the four groups divided by the presence of diabetes mellitus and ESRD (Figure 4C).
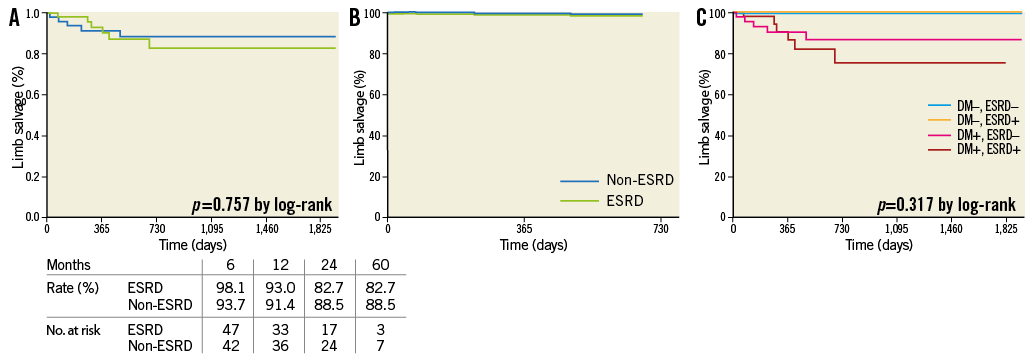
Figure 4. Limb salvage rate. A) Kaplan-Meier curves. There was no significant (p=0.757) difference between the two groups. B) Cox adjusted analysis. There was no significant difference between patients with and without ESRD. C) Post hoc Kaplan-Meier analysis.
As for AFS, Kaplan-Meier curves showed a significant difference (p=0.014, by log-rank) between the two groups (Figure 5A). The Cox model also identified ESRD (p=0.020) as an independent predictor of AFS (Figure 5B). In particular, there was a 124% increase in hazard ratio for death or major amputation for CLI patients with ESRD (Table 4). In addition to age (p=0.002), heart failure (p=0.005) and infectious wounds (p=0.015) were independently associated with AFS. According to a post hoc Kaplan-Meier analysis, there was a significant difference (p=0.027) in AFS among the four groups divided by the presence of diabetes mellitus and ESRD (Figure 5C).
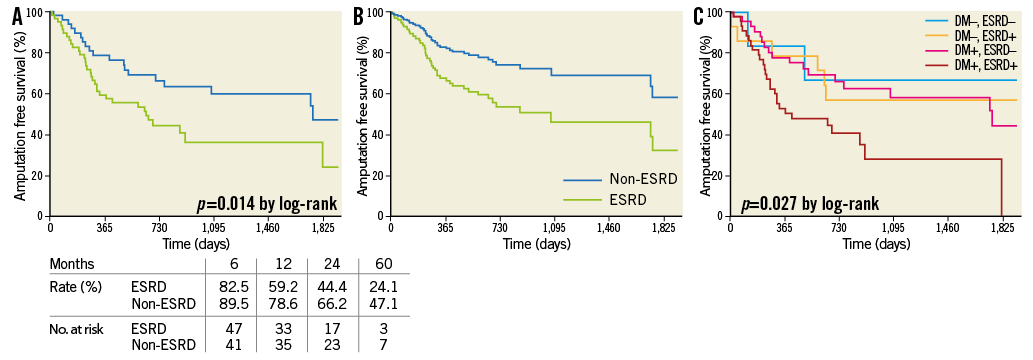
Figure 5. Amputation-free survival rate. A) Kaplan-Meier curves. There was a significant (p=0.014) difference between the two groups. B) Cox adjusted analysis. ESRD yielded a trend (p=0.064) towards lower AFS. C) Post hoc Kaplan-Meier analysis. There was a significant difference (p=0.027) among the four groups defined by the presence or absence of diabetes and ESRD. DM+, ESRD+ versus DM–, ESRD+; p=0.099 by log-rank. DM+, ESRD+ versus DM–, ESRD–; p=0.099 by log-rank. DM+, ESRD– versus DM–, ESRD+; p=0.688 by log-rank. DM+, ESRD– versus DM–, ESRD–; p=0.659 by log-rank. DM+, ESRD+ versus DM+, ESRD–; p=0.007 by log-rank. DM–, ESRD+ versus DM–, ESRD–; p=0.482 by log-rank.
Discussion
The main findings of this study were as follows: 1) ESRD patients were younger, but had more cardiovascular disease complications than non-ESRD patients; 2) ESRD patients were more likely to have distal artery disorders than non-ESRD patients; and 3) ESRD patients yielded a lower wound healing rate, higher reintervention rate and lower AFS rate than non-ESRD patients.
Cardiovascular risk in ESRD patients is five to 30 times that of the general population16. In this study, we found that ESRD patients presenting with CLI were younger and more likely to experience complications of heart failure and coronary artery disease requiring revascularisation. These findings may explain the more accelerated atherosclerosis and the more advanced systemic cardiovascular disorder in ESRD patients. Also, infrapopliteal artery disease is reported to involve long and/or occlusive atherosclerotic lesions17. Above all, infrapopliteal artery disease in ESRD patients is often marked by a severely diseased pedal arch, with one study showing a pedal arch occlusion rate of 58%18. The present study demonstrated a distinct difference between ESRD and non-ESRD patients in terms of the extent of infrapopliteal artery disease. As shown in Table 3, above-the-ankle lesions were less occlusive and less frequent in ESRD patients than in non-ESRD patients. In below-the-ankle segments, severe pedal arch disease, defined by a type 3 pedal arch equivalent to dorsal and plantar arch occlusion, occurred significantly more frequently in ESRD patients than in non-ESRD patients (52.3% vs. 32.7%). These findings suggest that ESRD patients are more susceptible to distal vascular disorders than non-ESRD patients.
Surgical bypass studies have reported 30-day mortality rates of 8.8-18% in ESRD patients but only 3.1% in non-ESRD patients7-10. Also, in the treatment of CLI patients with ESRD, bypass surgery resulted in four times the amputation rate, 1.37 times the all-cause mortality, 1.50 times the cardiac mortality, and 2.17 times the infectious mortality compared with endovascular therapy8. On the other hand, a recent endovascular study reported 30-day mortality rates of 8% in CLI patients with ESRD19. In this study, the 30-day mortality of 3.5% in ESRD patients was higher than in non-ESRD patients but not significantly so. These findings suggest that, although endovascular intervention in the treatment of ESRD patients could be safer than surgical intervention, 30-day mortality in ESRD patients might still be higher than in non-ESRD patients, possibly even in the setting of catheter-based infrapopliteal intervention.
Although increased attention has been paid to wound healing as a practical endpoint20, scant data are available especially in patients with ESRD. According to a recently published surgical bypass study21, the wound healing rate was approximately 60-80% at one year in ESRD patients as compared to approximately 90% in non-ESRD patients. In the present study, the wound healing rates (66.7% in ESRD patients, 81.6% in non-ESRD patients, p=0.028) were comparable to those in surgical studies. However, according to the Cox model, ESRD patients had approximately twice the risk of the wound not healing as non-ESRD patients. This finding might be due to ESRD-specific malnutrition and insufficient microcirculation. Also, despite liberal debridement, the Cox model identified infectious wounds, diabetes mellitus and the severity of pedal arch (pedal arch classification) as independent predictors of wound healing. Indeed, according to a post hoc Kaplan-Meier analysis, wounds stratified by the presence or absence of diabetes mellitus and ESRD presented distinct healing rates. These findings suggest that a more proactive multidisciplinary approach of different disciplines and clinically driven pedal artery intervention are further requisites for wound healing, especially in patients with ESRD.
According to Baumann et al, the clinically driven reintervention-free rate in CLI patients with a mean serum creatinine level of 151.6±159.7 µmol/L was 29.1% at six months and 41.6% at 12 months22. This finding is consistent with the reintervention rates on non-ESRD patients in this study (31.5% at six months and 39.4% at 12 months). However, reintervention rates in ESRD patients were significantly higher (56.9% at six months and 59.9% at 12 months) than those in Baumann et al’s study and in this study’s non-ESRD patients. Even after Cox model adjustment for confounding factors, ESRD patients had approximately twice the risk of reintervention as compared to non-ESRD patients. Although no independent predictors of reintervention were observed in Baumann et al’s study, the Cox model in this study found that ESRD was an independent predictor of reintervention, albeit the only one. The reasons that reintervention may be required in ESRD patients might be due to ESRD-specific vascular disorders including an aggressive atherosclerotic process, development of vessel calcification and impaired vascular resistance4-5. Therefore, the benefits offered by antirestenosis technology in the field of infrapopliteal intervention might be a greater boon to ESRD patients than to those without.
As for limb salvage rates after infrapopliteal angioplasty, Brosi et al reported limited efficacy of balloon angioplasty in ESRD patients, with a 73% one-year limb salvage rate18. However, Aulivola et al reported significantly lower limb salvage rates in ESRD patients than in non-ESRD patients (52.5% vs. 84.4% at one year, 52.5% vs. 80.2% at three years, p=0.01)23. In the present study, no significant difference in the limb salvage rate was observed between patients with and without ESRD. Given the poor AFS in ESRD patients, it is noteworthy that the finding of comparable sustained limb salvage rates in ESRD and non-ESRD patients is due to poor survival and the effects of the competing mortality hazard, especially in ESRD patients. Also, based on the Cox model’s findings and post hoc Kaplan-Meier analysis, ESRD (especially diabetic ESRD) patients with heart failure and infectious wounds could be at the highest risk for major amputation or death after intervention.
Limitations
Several limitations must be considered. First, the present study used a relatively small sample size in a single centre and was retrospective in design. Therefore, because of the potential of low statistical power for detecting difference between the two groups, a type II statistical error might exist. Second, there is the possibility of population selection bias. However, with the almost unlimited availability of medical care in Japan due to a unique healthcare and reimbursement system, individuals with ESRD account for a large percentage of CLI patients. Indeed, this study included nearly all consecutive patients requiring infrapopliteal revascularisation at a clinical practice, and approximately 50% of this study’s population suffered from ESRD complications and received haemodialysis. Third, this study did not take into account currently available endovascular technology, including drug-eluting stents or drug-coated balloons, although the cost-benefit analysis of these novel technologies is not yet complete24.
Conclusions
In conclusion, ESRD patients yielded a more affected pedal arch, and were at approximately twice the risk of wound healing failure, reintervention, and death or major amputation than non-ESRD patients. We hope that these findings can be used to improve the quality of CLI treatment as part of a coordinated approach involving cutting-edge endovascular revascularisation and wound management.
| Impact on daily practice Very few data are available on the performance of infrapopliteal interventions for patients with critical limb ischaemia (CLI) accompanied by end-stage renal disease (ESRD) in real-world practice. This study demonstrated that, among CLI patients, those with ESRD have a more affected pedal arch. Despite higher rates of clinically driven reintervention, ESRD patients remain at approximately twice the risk of non-healing wounds, death, or major amputation compared to CLI patients without ESRD. These findings suggest that antirestenosis technology and an interdisciplinary approach are necessary for the treatment of CLI patients, especially those with ESRD. |
Acknowledgement
This article was supported in part by grants-in-aid for Scientific Research C (Grant Number 25461096).
Conflict of interest statement
The authors have no conflicts of interest to declare.

