CASE SUMMARY
BACKGROUND: An 84-year-old man suffering from dyspnoea on mild exertion and a 10-year history of mitral valve replacement with a mechanical prosthesis presented to our department. The patient had an isolated right aortic arch.
INVESTIGATION: Transthoracic echocardiography demonstrated severe LV systolic dysfunction (EF 25%), good function of the previously implanted mechanical prosthesis and severe aortic stenosis. Multislice computed tomography confirmed the presence of an isolated right aortic arch with mirror-image branching.
DIAGNOSIS: Severe symptomatic aortic stenosis in a patient with right aortic arch at high risk for surgical reintervention.
MANAGEMENT: Transcatheter aortic valve implantation using conventional delivery system.
KEYWORDS: Edwards prosthesis, mirror-image right aortic arch, severe aortic stenosis, TAVI technique, transfemoral approach, tricks in TAVI
PRESENTATION OF THE CASE
An 84-year-old man, suffering from dyspnoea on mild exertion (New York Heart Association functional Class III) and a 10-year history of mitral valve replacement with a mechanical prosthesis, presented to our department. The previous pre-intervention angiography highlighted the presence of a right aortic arch (Moving image 1). Comorbidities included hypertension, diabetes mellitus with insulin treatment, permanent atrial fibrillation, chronic renal failure, congestive heart failure, and hypercholesterolaemia. The logistic EuroSCORE was 56% and the Society of Thoracic Surgeons score was 35%. Transthoracic echocardiography demonstrated increased left ventricular (LV) systolic and diastolic volumes, severe LV systolic dysfunction (EF=25%) and a good function of the previously implanted mechanical prosthesis with a mitral valve area by pressure half-time of 3.0 cm2 and no paravalvular leaks. Aortic valve was severely calcified, the mean transvalvular gradient was 38 mmHg and effective orifice area was <1.0 cm2. Pharmacologic (dobutamine) stress echocardiography demonstrated the presence of LV contractile reserve with an increase of the mean gradient to 45 mmHg and an effective orifice area staying at <1.1 cm2. The anatomy of aortic root, aortic annulus, and aortic arch was assessed by multislice computed tomography, confirming the presence of an isolated right aortic arch with mirror-image branching (Figure 1A, Figure 1B). Congenital intracardiac and extracardiac diseases which could be possibly associated with this anomaly were excluded. Aortic annulus size measured 24 mm. No critical stenosis of epicardial coronary arteries was demonstrated at coronary angiography. The patient was considered as high risk for classical surgical reintervention. Thus, the Heart Team considered this patient not a surgical candidate and he was referred for transcatheter aortic valve implantation (TAVI). Potential technical options to be adopted for TAVI included:
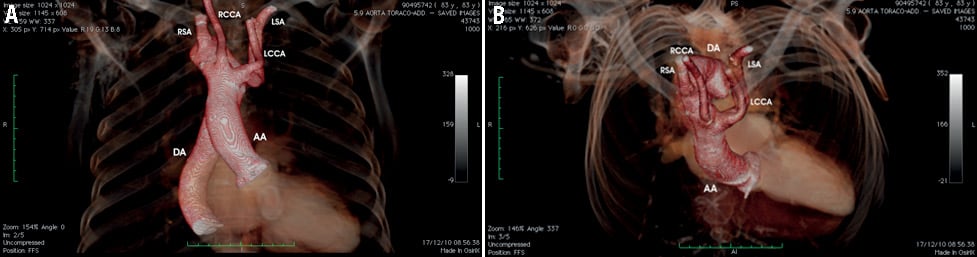
Figure 1. Multislice computed tomography demonstrating the mirror-image right aortic arch. This is the left-right mirror of a normal left aortic arch. (A) Mirror-image right arch has the first branch as a left innominate artery (which, in turn, divides into left carotid and left subclavian arteries), the second as the right carotid, and the third a right subclavian, as clearly demonstrated in panel B. AA: ascending aorta; DA: descending aorta; LCCA: left common carotid artery; LSA: left subclavian artery; RCCA: right common carotid artery; RSA: right subclavian artery
1) transfemoral approach using a modified technique and a device with high navigability through the aorta;
2) transapical approach;
3) transaortic approach.
How would I treat?
THE INVITED EXPERTS’ OPINION
Right-sided aortic arch (RAA) is the most common arch anomaly in paediatric patients. It has been suggested a 1 of 1,000 pregnancy rate in a low-risk population. The outlook for these patients largely depends on the presence of associated congenital heart defects and physiologic abnormalities, which include tracheobronchial compression, oesophageal compression and abnormal flow patterns. If isolated, aortic arch anomalies are asymptomatic vascular variants in most cases.
D’Ascenzi and colleagues report the case of an 84-year-old man with RAA, previous mitral valve replacement with a mechanical prosthesis, chronic renal failure, severe LV systolic dysfunction and a symptomatic severe aortic stenosis who was not considered a surgical candidate and therefore referred for transcatheter aortic valve implantation (TAVI). Multislice computed tomography images (Figure 1A, Figure 1B) and aortography (Moving image 1) show a tricuspid aortic valve with moderate calcifications and a mildly tortuous RAA and descending aorta. Unfortunately, we do not have data about abdominal aorta, iliac and femoral arteries. Current literature does not consider vessel tortuosity as an absolute contraindication to TAVI, since stiff wires and special techniques such as the “railing track” (used with success in the field of endovascular aortic aneurysm repair)1,2 can straighten the arteries enough to permit the advancement of the introducer and the delivery system through the iliac vessels and the aorta. Given this information, this case does not seem to present any anatomic characteristics that preclude the use of the transfemoral approach although the non-standard anatomy and angiographic aspect might make the optimal alignment of the aortic cusps and the fine positioning of the prosthesis more difficult and time-consuming. As far as prosthesis type is concerned, both balloon-expandable (Edwards SAPIEN XT®; Edwards Lifesciences, Irvine, CA, USA) and self-expandable (Medtronic CoreValve®; Medtronic, Minneapolis, MN, USA) valves seem to be suitable for implantation. The presence of a mitral mechanical prosthesis and aortic arch tortuosity could favour the use of an Edwards prosthesis with the NovaFlex™ delivery system (also Edwards Lifesciences). On the other hand, the absence of severe calcifications of the native valve and the absence of information about the distance of coronary ostia from the annulus suggest the use of the CoreValve prosthesis. The operator’s clinical experience together with an accurate analysis of CT data will be crucial for the identification of the appropriate device.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
Our preferred approach is the transfemoral, pending view and measurements of the iliofemoral arteries and abdominal aorta scans. The transfemoral approach is less invasive than the transaortic and transapical approaches. Previous sternotomy may prohibit surgical access. The left transaxillary approach could also be challenging in the presence of the right-sided aortic arch.
We would perform the transfemoral approach under conscious sedation and local anaesthesia. This allows early mobilisation and discharge. The patient does not present with specific risks that mandate general anaesthesia.
We recommend transoesophageal echocardiography (TOE) guidance with conscious sedation and anaesthetic support. In our experience, this is well tolerated. TOE allows confirmation of detailed valvular anatomy including coronal and sagittal annular diameters, guides aortic valve crossing/balloon aortic valvuloplasty/prosthetic deployment, and diagnosis and rescue of complications.
Our routine strategy would be to preclose the TAVI femoral arterial access with two ProGlide (Abbott Vascular, Santa Clara, CA, USA) devices (pending aorto-iliofemoral data). The choice between left and right femoral TAVI access will depend on the iliofemoral data. A 5 Fr sheath is inserted in the contralateral femoral vein for temporary pacing of the right ventricle. A 5 Fr sheath is inserted in the contralateral femoral artery for the monitoring angled pigtail catheter.
On the basis of the aortic valve annulus of 24 mm (following validation by periprocedural TOE), we would choose a 26 mm Edwards SAPIEN XT® (Edwards Lifesciences, Irvine, CA, USA) or 29 mm Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) TAVI prosthesis. The aortic root and ascending aorta appear elongated and angulated at about 30 degrees to the vertical plane on the CT data; a balloon-expandable valve with deflecting mechanism may allow better coaxial alignment during deployment. The position and height of the coronary arteries above the annulus – which may be anomalous in the right-sided aortic arch – must be verified on TOE or angiography, in the context of the minimum coronary height requirement of different technologies. The final choice between commercially available devices is based on detailed assessment of iliofemoral access. If a balloon-expandable prosthesis is used, the operator will need to operate the delivery handle upside down and without direct visualisation of the buttons; vigorous fluoroscopic attention to the loading of the valve onto the balloon is required.
Particular attention should be paid to the positioning and manipulation of the stiff guidewire within the right-sided aortic arch. The optimal fluoroscopic plane for TAVI will be chosen from 3D CT analysis and adjusted during procedural angiography. The CT data suggest that RAO fluoroscopy with a craniocaudal tilt may be ideal for TAVI, in providing an aligned view of the coronary cusps, the ascending aorta and arch. PA fluoroscopy may be required to confirm coaxial deployment of the prosthesis.
Following TAVI, all arterial access sites would be closed with standard closure devices to enable early mobilisation of the patient. The patient would be transferred to a monitored bed with planned discharge in 72 hours.
Conflict of interest statement
J. Kovac is a proctor for CoreValve Medtronic, Edwards Lifesciences, a trainer and consultant at St. Jude Medical, an investigator for St. Jude Portico, CoreValve Advance, and a member of the Steering Committee Advance Plus. D.T. Chin is a co-investigator at CoreValve Advance and St. Jude Portico. J.H. Baron has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The procedure was carefully planned with the decision to implant, through a right transfemoral approach, a 26 mm Edwards SAPIEN® aortic valve (Edwards Lifesciences, Irvine, CA, USA). We used a left femoral artery approach and balloon valvuloplasty was performed, under rapid ventricular pacing. While advancing the delivery system at the level of the distal transverse aortic arch, during the first step of the navigation, the NovaFlex® delivery system (Edwards Lifesciences) was flexed in the opposite to standard direction, with the “E” mark lying down (Moving image 2, step 1). This technique allowed us to have a smooth aortic arch tracking. After the arch was crossed, the delivery system was unflexed and the “E” mark returned to the recommended position; then the native valve was crossed without difficulties (Moving image 2, step 2, Moving image 3). Thus, once the coaxial position was achieved, the valve was successfully implanted (Figure 2). The delivery system was removed without producing aortic injuries (Moving image 4).
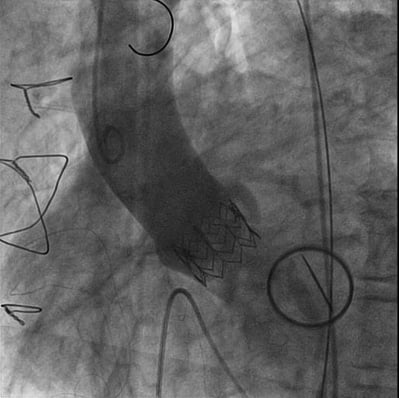
Figure 2. Final angiogram demonstrating the implantation of the Edwards SAPIEN® device. 52° left anterior oblique, 4° cranial view.
Transoesophageal echocardiography confirmed the proper positioning of the prosthesis at the aortic annulus, with the presence of a post-procedural paravalvular leak 2+/4+, due to a bulky leaflet calcification of the native aortic valve. The femoral access was closed by a percutaneous closure system. In-hospital stay was uneventful, and the patient was discharged home shortly afterwards. A three-month clinical and echocardiographic follow-up, performed as per protocol, showed an improved quality of life with a substantial improvement of NYHA functional class, an improvement of LV systolic function (EF 40%), and good prosthesis function with a mild aortic paravalvular leak, a peak gradient of 18 mmHg, and a mean gradient of 9 mmHg.
Discussion
TAVI has evolved into a feasible therapeutic option for the management of selected patients with severe aortic stenosis and at high or prohibitive risk for standard surgery3,4. As the procedure moves more into mainstream use, more successful solutions of challenging cases are being reported5. The configuration and the anatomy of the aortic arch differ in each patient and sometimes specific variations may occur. Isolated right aortic arch is one of them, and is an abnormal regression of the left eighth dorsal segment resulting in an aortic arch that crosses the right mainstem bronchus and passes to the right of the trachea. Because of specific characteristics and considering that conventional delivery systems are mostly designed for left aortic arch, right aortic arch can represent a challenge for the interventional cardiologist in obtaining procedural success and avoiding periprocedural complications.
The complex anatomy of a mirror-image right aortic arch requires a controlled orientation of the delivery system in the three directions during TAVI. We demonstrated the feasibility of performing a TAVI in patients with right aortic arch using a conventional delivery system, suggesting a trick to perform a percutaneous aortic implantation in this particular setting.
In this case we used the Edwards NovaFlex™ transfemoral delivery system because it facilitates guidance over the aortic arch with its articulating distal end and allows a more controlled navigation. The trick to perform the first step of the procedure with a reverse position of the “E” mark of the delivery system allowed us to cross the arch successfully without difficulties, avoiding the accumulation of tension on the delivery system during the navigation over the aorta. Furthermore, we demonstrated that the flexing and deflexing manoeuvres did not cause kinking or torsion of the delivery system.
Whether TAVI could be performed using different devices or different vascular approaches in patients with right aortic arch needs to be determined in future reports. In this case, as in all patients with LV dilatation and severely impaired LV systolic function, we preferred to choose a transfemoral rather than a transapical approach considering the potential greater degree of myocardial damage with the latter approach.
A careful preprocedural evaluation of patient candidates for TAVI plays a relevant role in optimising the procedure, and detailed information on aortic annulus, peripheral access and aortic arch anatomy are critical for a successful implantation, guiding the selection of the device6. After several years’ worth of experience, we find that TAVI procedures are becoming more routine. Patient referrals are growing and the demand for TAVI procedures is escalating, but we need to increase our practice safely while maintaining the original level of attention and quality of care. In this perspective, sharing information regarding the management of challenging cases is an important issue.
Conflict of interest statement
G. Sorropago is a proctor for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Aortography demonstrating the presence of a mirror-image right aortic arch. 50° right anterior oblique, 1° caudal view.
Moving image 2. Intraprocedural moving image demonstrating the technique used to obtain a more controlled navigation over the aorta.
Moving image 3. Aortography showing the unusual navigation through the aorta. 50° right anterior oblique, 2° cranial view.
Moving image 4. Final result after transcatheter aortic valve implantation. 50° right anterior oblique, 1° caudal view.

