Abstract
Percutaneous implantation with the Melody® valve (Medtronic Inc., Minneapolis, MN, USA) has achieved standard of care for the management of patients with dysfunctional right ventricle (RV) to pulmonary artery (PA) conduits. The right ventricular outflow tract (RVOT) landing zone dimensions may vary significantly making it unfavourable for Melody valve implantation. We report a novel technique of “folded Melody valve” in a patient with complex, dysfunctional RV to the PA conduit.
Introduction
Over the last decade, percutaneous pulmonary valve implantation (PPVI) has emerged as a safe and effective alternative to open heart surgery and is now considered the treatment of choice for patients with a haemodynamically dysfunctional right ventricular outflow tract (RVOT)1,2. An unfavourable RVOT anatomy continues to be the Achilles heel, limiting the indications for PPVI. Interest in reducing the amount of surgery in patients with congenital heart defects is gaining momentum. Such RVOT constraints may sometimes lead to repeated open heart surgery with increased morbidity and mortality. In patients with a large RVOT, there are reports of advanced techniques which render these patients amenable to the transcatheter technique (i.e., use of two Melody valves implanted in respective pulmonary arteries in a single patient; jailing and/or Russian doll technique)3,4. In patients with a short RVOT, the insertion of a Melody valve may not be possible because of the risk of jailing one PA if the Melody valve is positioned too high in the outflow. The risk of stent fracture is also possible if the position of the valve is too low in the contractile RVOT or behind the sternum. In that situation, there is a need for a valve shorter than the Melody valve or for the creation of a safe and stable, subannular platform by implanting multiple bare metal stents in the vertical retrosternal portion of the RVOT. The subannular position may not be ideal, and pre-stenting may reduce but not eliminate the risk of compression. The Edwards valve (Edwards SAPIEN Transcatheter Heart Valve; Edwards Lifesciences, Irvine, CA, USA) is an option; however, it may not be widely available. We describe a new modification of the Melody valve in a patient with a short complex RVOT.
Presentation of the case
A 10.5-year-old male with aortic atresia and ventricular septal defect came to our attention for RVOT obstruction. He underwent multiple surgical RVOT conduit revisions over the years (16 mm Contegra conduit [Medtronic, Inc.] followed by an 18 mm Shelhigh [Shelhigh, Inc., Millburn, NJ, USA]). Three years later, there was a recurrence with incompetence and stenosis requiring RVOT dilatation and stenting of the left PA (CP stent, 8Z22; NuMED, Inc., Hopkinton, NY, USA) (Figure 1). Follow-up echocardiography after three years showed severe RVOT obstruction with estimated RV systolic pressure of 100 mmHg by tricuspid regurgitation jet with a peak velocity of 4.5 m/sec across the RVOT. A cardiac CT scan showed mildly dilated and hypertrophied RV with RVOT length of 20 mm. A bare metal stent (LD Max™, 26 mm; ev3, Plymouth, MN, USA) was mounted on a 20 mm BIB balloon (NuMED) and deployed in the RVOT with its distal end anchored in the proximal LPA (Figure 2). The landing zone for the Melody valve consisted of a vertical retrosternal part followed by a horizontal part measuring 18 mm in length. The insertion of the Melody valve in the proximal part would have required reinforcement with a new bare metal stent to prevent but not eliminate the possibility of retrosternal compression. Moreover, there was a risk of Melody valve impingement with the RV muscle at the proximal end. Given the problems precluding proximal deployment and the risk of accidental covering of the PA distally, the effective landing zone of the Melody valve was shortened. To circumvent these potential complications, we decided to modify the Melody valve before implantation. The terminal open stent struts on either side of the Melody valve were folded over themselves from the inside out to reduce its effective length on a 10 ml syringe (Figure 3). Despite this slight modification, the crimping and loading of the Melody valve was performed using the usual technique. The folded and crimped valve moved easily in the 22 mm delivery system and the deployment was performed using the standard technique with a stiff wire in the left PA. The Melody valve was placed slightly below the previous CP stent to avoid accidental covering of the right PA. The wire was then repositioned through the mesh of the stents (CP and ev3) in the right PA and an Atlas® balloon (Bard Peripheral Vascular, Inc., Tempe, AZ, USA) was inflated to open the cells of the stents (Figure 4). There was no paraprosthetic leak, residual pulmonary regurgitation or pulmonary stenosis immediately after implantation. The patient was discharged after two days and a predischarge transthoracic echocardiogram showed maximal velocity across the RVOT of 2.4 m/sec. The patient remained asymptomatic after eighteen months of follow-up with an undisplaced and competent Melody valve except for trivial valvar regurgitation. No stent fracture was seen on fluoroscopy (Figure 5).
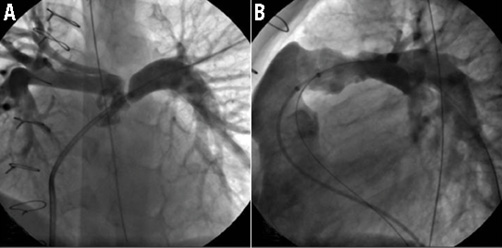
Figure 1. Still cine frames showing: A) ostial stenosis of the left pulmonary artery; B) short horizontal landing zone and free pulmonary regurgitation. LPA stenting was performed three years prior to Melody valve implantation (uncovered CP stent). Panels (A) and (B) were obtained from that previous cardiac catheterisation to demonstrate the RVOT anatomy better.
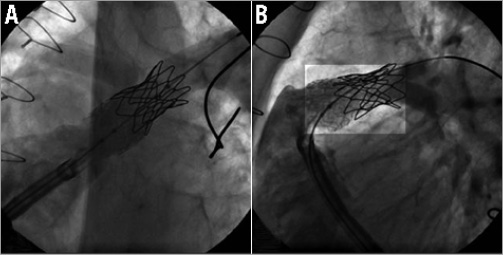
Figure 2. Still cine frames showing pre-stenting of the RVOT with an ev3 LD Max (26 mm). A) The stiff wire is parked in the LPA. BIB balloon is inflated to expand the BMS in the RVOT. B) Dye injection after RVOT pre-stenting showing the position of the ev3 stent. Note the difference of radiopacitiy between the CP stent placed three years previously and the ev3 stent.
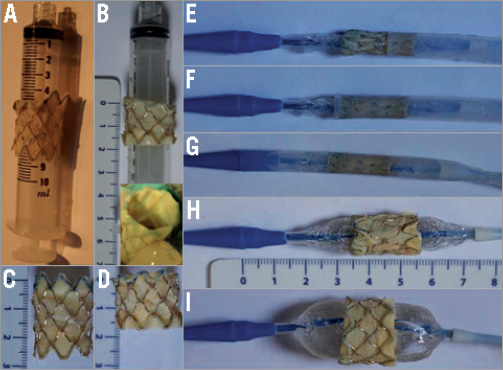
Figure 3. In vitro pictures showing the folding of the Melody valve during bench testing, reproducing what is done in patients. A) The folding is shown in process. The folding is performed on a 10 ml syringe. In vitro appearance after folding of two terminal stent struts of the Melody valve. B) In vitro appearance of the completely folded Melody valve (upper panel). Note there is no disruption of cusp anchor or leaflets (lower panel). C) and D) Melody valve is shown in its unfolded (C) and folded configurations (D). Note the significant shortening of the device. E) - G) The crimping of the Melody valve and preparation of the delivery catheter are done using the conventional technique. Balloons of the Ensemble catheter should not be inflated during preparation. Inflation of the balloons would make crimping of the folded Melody valve more difficult. The folded Melody valve is shown at different stages of loading: E) the first row of the Melody valve is covered; F) the folded Melody valve is entirely covered; G) the Melody valve is totally mounted in the Ensemble catheter ready to be inserted in the patient. H) and I) Simulations of balloon inflations of the Ensemble catheter. H) Inner balloon has been inflated. I) Outer balloon is totally inflated.
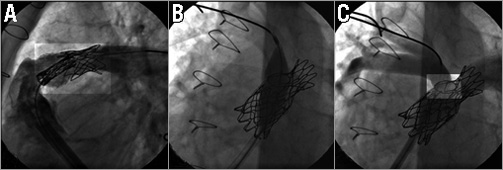
Figure 4. Still cine frames showing: A) the undeployed folded Melody valve advanced and positioned in the pre-stented RVOT in the 22 mm Ensemble delivery system. The wire is in the LPA. To avoid accidental RPA covering, the Melody valve is distally placed just below the previous CP stent that covers the RPA ostium. Proximally, the Melody valve is positioned as far as possible in the pre-stented region. Note the increase of radiopacity at both extremities of the folded Melody valve. B) The wire has been repositioned in the RPA and a high-pressure balloon is shown inflated in the ostium of the RPA to open the struts of the CP and ev3 stents. C) Final angiogram after Melody valve deployment and opening of the stent struts covering the right PA showing a competent Melody valve with no valvar or paravalvar leaks. The folded Melody valve is well seated distally and away from the sternum. The struts of the stents at the level of the RPA ostium are opened widely giving a round shape of the cells.
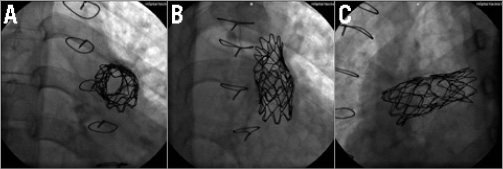
Figure 5. Still cine frames from fluoroscopy performed at the last follow-up showing no stent fracture.
Discussion
We tested the feasibility of using a Melody valve which had been shortened by hand by folding the terminal stent struts from inside out at both ends. By doing so, i) the number of rows of the stent dropped from six to four; ii) this allowed a clear and sharp demarcation of its proximal and distal ends. The length of a CP8Z34 (six rows) expanded on a 22 mm balloon is 24.6 mm. The length of a CP8Z22 (four rows) is 16.7 mm when expanded on a 22 mm balloon giving a reduction in length of 7.9 mm. Most importantly, by folding the ends of the Melody valve there was no compromise on the integrity and competence of the cusps noted both in vitro as well as in vivo. Moreover, there was re-enforcement of the stent struts giving additional support. However, by folding the ends of the stent, stent integrity could be theoretically compromised and lead to an increase of stent fracture at this level. If this happened, it would lead to separation of the folded rows from the Melody valve giving rise to two different stents but physically still attached by stitches and the venous wall of the Melody valve. In addition, these rows trapped between the wall of the conduit and the Melody valve would not embolise and, in our opinion, this would have very limited impact.
The valve should be folded over a 10 ml syringe before crimping. The folded valve should then be crimped on a 5 and a 2.5 ml syringe and finally on a 22 mm Ensemble delivery system (Medtronic, Inc., Minneapolis, MN, USA) without difficulty. Due to the shortening of the folded Melody valve, one should be careful as to how and where to crimp the folded valved stent on the outer balloon, keeping the RVOT and PA anatomy in mind. In our experience, with early PA bifurcation and relatively small PAs, the Melody valve should be mounted at the most distal end of the balloon. By doing so, one may avoid shouldering of the balloon in the branch PA. Failure to do so may cause Melody valve displacement during inflation. Other than this modification, the Melody valve was prepared and deployed using the standard technique in this case. The movement of the folded valve in the delivery system was smooth and without any difficulty. There may be a theoretical possibility of the valve getting wedged in the delivery sheath during deployment. However, we did not encounter such a situation. Immediate post-procedure angiography showed a competent Melody valve with no impingement on the RV proximally or the PAs distally. One could argue that this case could have been dealt with using the conventional method. However, using this technique, we were able to stay away from the retrosternal part of the RVOT which has been shown to be one of the strongest predictors of stent fractures5. This does not necessarily imply that this technique would lead to a reduction of stent fracture.
The folded Melody valve technique is a practical approach for patients with short, complex RVOT, as described here. The shortening of the Melody valve is maximal by folding the two extremities. More folding would lead to dysfunction of the valve and is not recommended. However, in a situation requiring less shortening, only one row may be folded (proximal or distal).
Indications for pre-stenting the RVOT remain unchanged with the folded technique despite stent reinforcement of the extremities caused by the folding. The pre-stenting remains, in our opinion, necessary to reduce the risk of stent fracture at least in patients with unsupported conduits. In the future it may be possible to extend this technique to patients with critical and challenging RVOT neighbouring anatomy as seen with early PA bifurcation or close proximity of the left main coronary artery that may preclude a conventional technique and compel referral for surgery. In such cases, a shortened Melody valve as described may be positioned further away from the vulnerable coronary artery, avoiding compression (at least theoretically).
The covering/uncovering of the Melody valve was fairly smooth. There were no stent-related complications noted after a follow-up period of eight months.
Conclusion
The “folded valve technique” is quite easy to understand and implement. The technique is a safe addition to the interventional armamentarium allowing the implanting physician to modify the valve in patients with complex short RVOTs. By implanting the Melody valve far from the sternum, this technique may reduce the overall incidence of stent fractures reported with this device. Of course, this remains speculative and needs to be proven with larger series and longer follow-up. In the future, this technique may also be a good option for patients with vulnerable RVOT surroundings that may preclude a conventional technique.
Conflict of interest statement
Y. Boudjemline acts as a proctor for Medtronic Inc. The other authors have no conflicts of interest to declare.

