
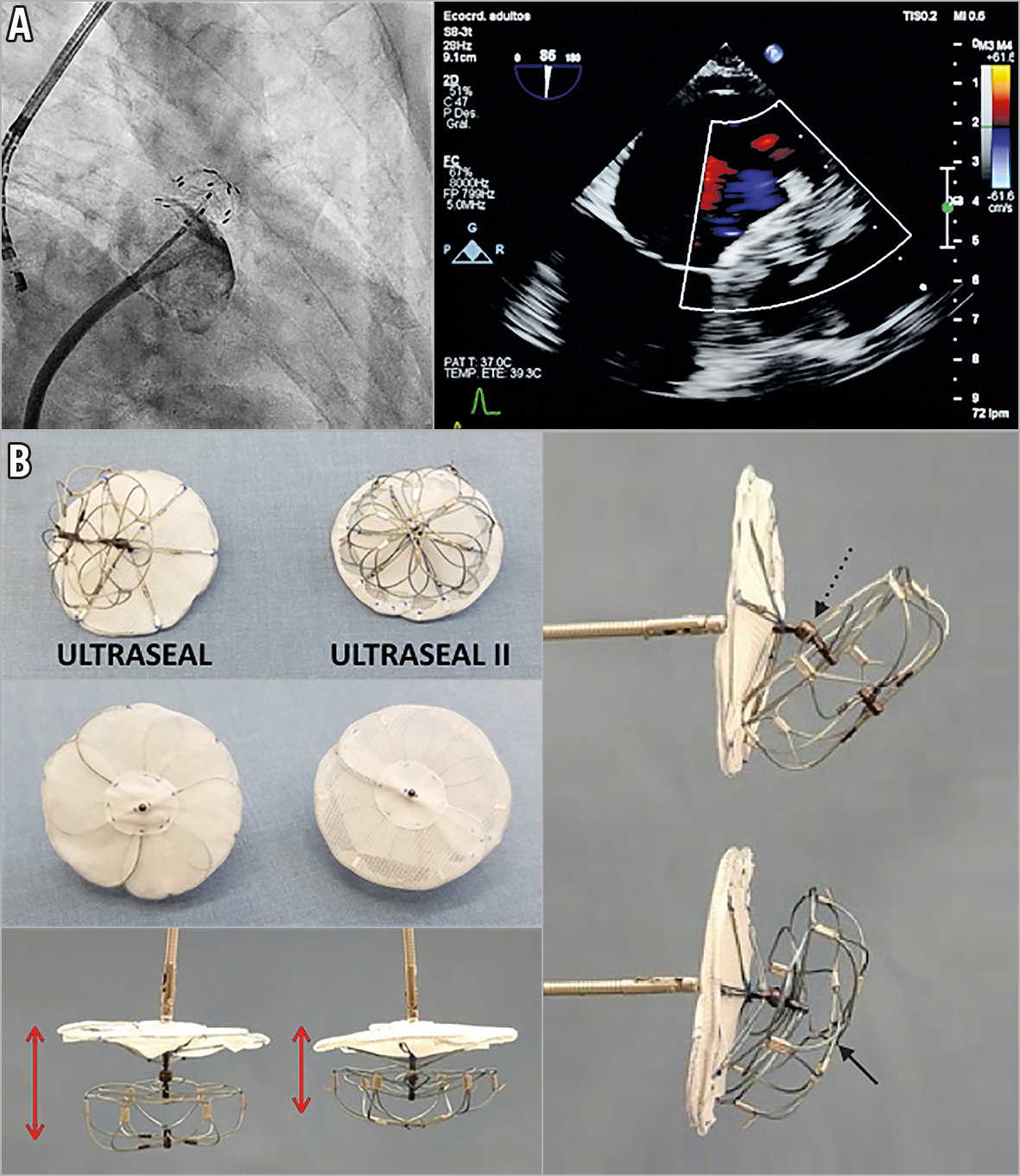
Figure 1. First-in-human experience with the Ultraseal II. A) LAA occlusion with the Ultraseal II device and (B) comparison between the first Ultraseal device and the new Ultraseal II.
A 74-year-old man with atrial fibrillation and spontaneous intra-cerebral haemorrhage was admitted for percutaneous closure of the left atrial appendage (LAA). We present the first-in-man experience with the Ultraseal II device (Cardia, Inc., Eagan, MN, USA).
Under conscious sedation, the procedure was carried out using transoesophageal echocardiography (TEE) with an S8-3t microprobe (Philips Medical Systems, Andover, MA, USA) and fluoroscopic guidance. A 22 mm Ultraseal II was selected. The sheath was positioned at the intended landing zone, then the bulb was deployed. Next, the sheath was retracted until the sail was deployed, achieving a complete sealing of the LAA (Figure 1A, Moving image 1-Moving image 3). Follow-up TEE showed no device-related thrombus and no residual leak.
The initial experience with this device has already been reported, with a high success rate and a low complication rate. However, modifications were warranted in order to facilitate deployment and retrieval and potentially to reduce the rate of device thrombosis (see Supplementary Table 1 for additional information). The Ultraseal II is a self-expanding nitinol device composed of two parts – the bulb, which secures the device position but does not seal the appendage, and the sail. These are connected by an articulating joint (Figure 1B, dashed arrow) that allows the sail to conform naturally to the LAA ostium. The new device is shorter, so the minimum required depth is smaller (Figure 1B, red arrows). The distal centre post has been removed (Figure 1B, black arrow), so the new device is more flexible. The joint is less articulated to enhance the anchoring of the bulb, without allowing the device to roll over easily, but being able to adapt to different LAA anatomies. The sail covering has been moved, the polyester coating is placed outside and the polyvinyl alcohol (PVA) inside, which makes it easier to load and retrieve the device. It will be necessary to evaluate whether this improvement could have an impact on the rate of thrombosis related to the device. The main distinctive features of the Ultraseal II compared to other disc-lobe devices are: the articulating joint that allows the sail to conform naturally to the LAA anatomy, the soft and flexible bulb, with low radial force, which allows a safe deep implant in the case of limited usable length and permitted oversizing if required, and the option of partial or total recapture up to five times.
In conclusion, the new device has the potential to adapt to different LAA morphologies and could be very useful in difficult anatomies.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Angiography evaluating the left atrial appendage morphology.
Moving image 2. Complete deployment of the Ultraseal II device.
Moving image 3. Angiography showing the final position of the Ultraseal II device and successful sealing of the LAA.

