Abstract
Aims: To evaluate a novel modality utilising ultrasound for renal denervation designed to reduce the duration of the intervention as well as to increase the consistency of the clinical outcome.
Methods and results: Eleven consecutive patients suffering from resistant hypertension as defined by the ESH-ESC guidelines were treated by transcatheter renal denervation using the CE-marked PARADISE™ technology (ReCor Medical, Ronkonkoma, NY, USA). An average of 5.1 ultrasound emissions were delivered in each subject for a total denervation duration of less than four minutes and the treatment was well tolerated by all patients. Both office and home blood pressure measurements showed an immediate, significant and sustained decrease in blood pressure. Three-month results were comparable to published data on radiofrequency renal denervation with an average reduction in office and home blood pressure of –36/–17 mmHg and –22/–12 mmHg, respectively.
Conclusions: Ultrasound renal denervation appears to be a safe and effective treatment for resistant hypertension and further studies will be performed to confirm these preliminary results.
Introduction
Hypertension significantly increases the risk of stroke, heart failure, chronic kidney disease and heart attack, posing serious health risks to those suffering from the disease. Sympathetic overactivity has long been recognised as one of the contributing factors for human hypertension. Several studies of experimental models of hypertension in both animals and human subjects have demonstrated that sympathetic overactivity plays a central role in hypertension.1 In addition, and consistent with the previous findings, in several animal models of experimental hypertension, bilateral renal denervation prevented the development or attenuated the magnitude of hypertension.2-6
Recently developed endovascular catheter technology enables selective denervation of the human kidney, with radiofrequency energy delivered in the renal artery lumen, accessing the renal nerves located in the adventitia of the renal arteries. Clinical studies have endeavoured to assess the safety and efficacy of a percutaneous, catheter-based approach designed to ablate renal sympathetic nerves specifically, using a radiofrequency generator via the lumen of the main renal artery (Simplicity™; Medtronic, Palo Alto, CA, USA). In a safety and proof-of-principle study, and in a separate randomised controlled trial, this approach was shown to reduce blood pressure successfully, without serious adverse events in patients with resistant hypertension.7,8 Durability of effect up to two years using this novel technique has recently been reported in a cohort of 153 patients with resistant hypertension treated with catheter-based renal sympathetic denervation at 19 centres in Australia, Europe and the United States.9 Recent publications have since confirmed the efficacy of radiofrequency renal denervation for the treatment of resistant hypertension.10
Although well received by the practitioners, current limitations of radiofrequency renal denervation relate to both the procedure itself and the success rate. Procedural limitations are inherent to the technical characteristics of the intervention and include the catheter instability which triggers frequent treatment interruptions regardless of the physician skills, the overall duration of the procedure which consists of a minimum of eight two-minute ablations, and the associated patient discomfort or pain necessitating sedation and analgesia with bradycardia reported in 13% of cases.11 Clinical limitations are revealed by the high percentage of non-responders as the mean decrease in blood pressure conceals considerable heterogeneity in individual responses. The inconsistency and unpredictability of the results is further aggravated by the absence of actual renal denervation direct measurement as the procedure offers no visibility on the treatment outcome during the intervention.12
The purpose of the REDUCE study was to evaluate the technical feasibility as well as the safety and efficacy of a novel modality for renal denervation designed to reduce the duration of the intervention as well as to increase the consistency of the clinical outcome.
Methods
Study setting
The clinical investigation plan for the REDUCE first-in-man study was approved by the Pharma-Ethics independent research ethics committee in South Africa. Patients were treated between October 2011 and February 2012 after signing a written informed consent at the Mediclinic Vergelegen, Somerset West, South Africa. Baseline and follow-up evaluation (scheduled at two weeks, one, two, three and six months) included physical examination, blood and urine analysis, and medication intake. Office (three measures), home (three measures twice a day during three days), and 24-hour ambulatory blood pressures were captured at regular time intervals during the course of the study. Baseline and follow-up (six-month) CT-scan images were reviewed by an independent radiological core laboratory. Patients were asked to keep on taking their antihypertensive medications unless otherwise indicated by the investigator.
Patient population
Patients were eligible to the REDUCE study if they were diagnosed with resistant hypertension as defined by the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC), i.e., with a minimum blood pressure of 140/90 mmHg (office), 135/85 mmHg (home) and 130/80 mmHg (ambulatory) despite being treated with at least three antihypertensive drugs including a diuretic.13 Patients under the age of 18 years, pregnant, allergic to contrast media, or with any known cause of secondary hypertension were excluded. Furthermore, a CT-scan was performed at screening to exclude patients with vascular abnormalities (including renal artery stenosis and iliac or femoral artery stenosis precluding insertion of the treatment catheter) or not meeting the anatomical criteria (renal artery of more than 20 mm in length and more than 4 mm in diameter).
Ultrasound treatment
Ultrasound energy consists of high-frequency sound waves (i.e., rapid mechanical oscillations), which are emitted by a piezoelectric transducer, pass through the surrounding fluids, and generate frictional heating in soft tissues resulting in temperature increase at depth. The CE-marked PARADISE™ technology (Percutaneous Renal Denervation System by ReCor Medical, Ronkonkoma, NY, USA) uses a catheter with a cylindrical transducer that emits ultrasound energy circumferentially.
The use of an energy that does not require direct tissue contact allows for a water balloon to be inflated around the transducer (Figure 1) and brings the following clinical benefits:
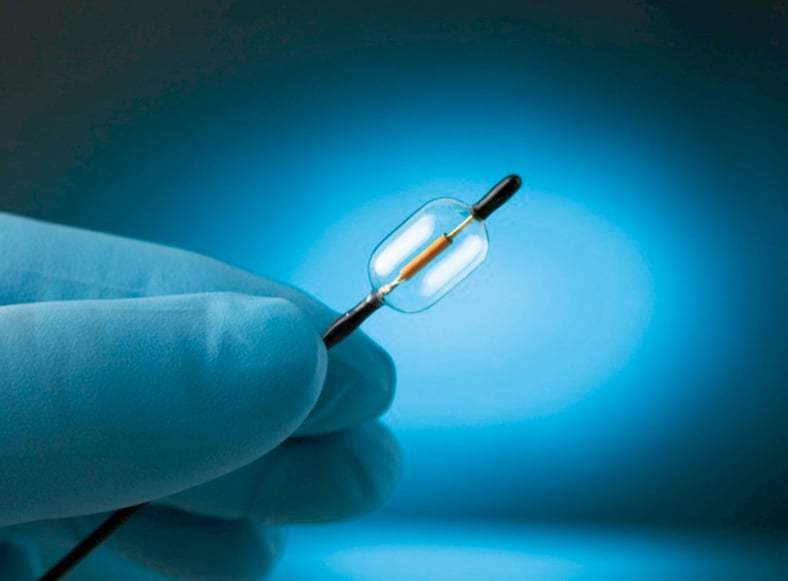
Figure 1. Tip of the PARADISE catheter showing the cylindrical ultrasound-emitting transducer encapsulated within an 8-mm diameter balloon.
– The balloon enables cooling fluid to circulate during the energy delivery process and keeps the arterial wall cool, minimising damage to non-target tissues
– The balloon positions the ultrasound transducer in the centre of the renal artery and enables uniform energy delivery circumferentially
– Controlled, uniform, and circumferential heating is independent of catheter positioning or tissue characteristics and increases the reproducibility of the procedure.
Interventions were performed under fluoroscopic guidance and the treatment catheter was introduced into each renal artery via femoral access. Bilateral denervation was achieved by delivering ultrasound energy in up to three locations within each renal artery starting in a distal position then pulling the treatment catheter back towards the ostium; each energy delivery consisted of one ultrasound emission at 25 or 30 watts for up to 50 seconds. Treatment settings (power and duration) were determined on the basis of the preclinical studies conducted on a cohort of 45 animals. Heparin was administered to the patients upon insertion of the arterial sheath in order to achieve a minimum of 250-second activated clotting time. Patients received sedatives (dexmedetomidine) and analgesics (sufentanil) during the intervention, and one injection of antispasmodic vasodilators (nitrates) was delivered in each renal artery prior to ultrasound emissions. Aspirin was given following the procedure at the dose of 100 mg daily.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation while frequency and percentage distributions were presented for qualitative variables. The statistical significance of the changes in blood pressure between baseline and follow-up was assessed using the Wilcoxon signed-rank test and a p value of less than 0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS version 17.0. Screening patient files including CT-scans and blood pressure measurements were collected electronically using decidemedical platform (ClinFlows, Hüllhorst, Germany).
Results
Patient characteristics
Eleven consecutive patients were enrolled and underwent renal denervation. Treated patients were 64% female and 36% male, 64% white and 36% coloured, and 55 ± 14 years old (range 32-80 years). Treated patients had numerous concomitant illnesses, including hyperlipidaemia (64%), coronary artery disease (36%), and diabetes (27%).
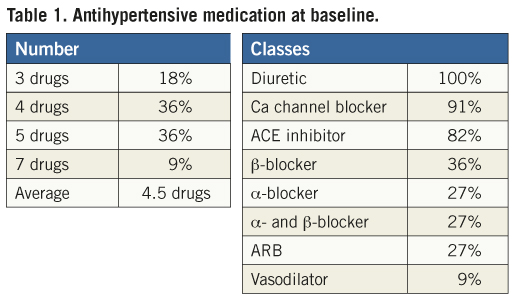
All patients met the ESH-ESC criteria for resistant hypertension at baseline. Average office blood pressure was 180±20/109±13 mmHg, average home blood pressure 169±14/101±13 mmHg, and average 24-hour ambulatory blood pressure 168±16/98±15 mmHg. Patients took, on average, 4.5 antihypertensive medications, with 100% receiving diuretics, 91% calcium-channel blockers, and 82% angiotensin-converting enzyme inhibitors (Table 1).
Treatment characteristics
A 12 Fr treatment catheter was used for the first three cases, while the following eight subjects were treated using a 6 Fr catheter (Figure 2). 12 Fr and 7 Fr introducing sheaths were used. Up to three ultrasound emissions were delivered in each renal artery, with an average of 5.1 ultrasound emissions per patient in total. None of the emissions exceeded 50 seconds and the average duration of each emission was 46 seconds, leading to a mean total emission time of less than four minutes per patient. On average, the elapsed time between the first and the last emission was 23 minutes per patient. Procedural pain was managed by the administration of sedatives and analgesics with no need for general anaesthesia during any of the interventions. One patient had renal artery dissection upon placement of the guiding catheter prior to the insertion of a 6 Fr treatment catheter, which was treated with a renal artery stent without any subsequent complication. This patient underwent denervation of the single contralateral renal artery. As previously reported following radiofrequency renal denervation, irregularities (or notches) and occasional spasms of the renal arteries could be observed by fluoroscopy after energy delivery. All patients were discharged on the day following the intervention with the exception of one subject, who experienced post-treatment hypotension, had to be hospitalised one more day, and was uneventfully discharged the day after.
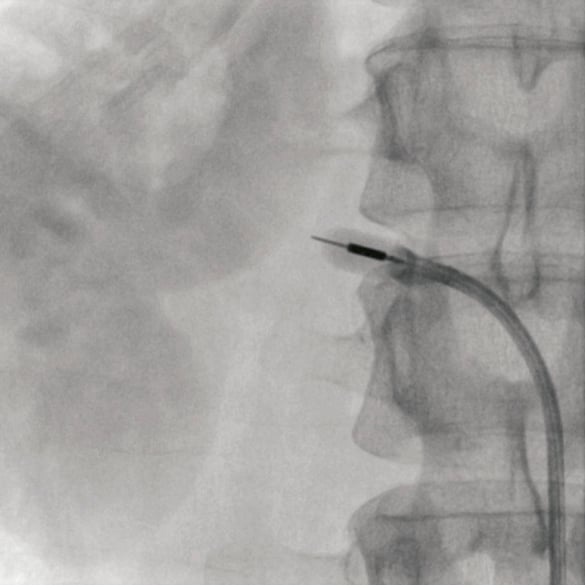
Figure 2. Angiography of the right renal artery showing the tip of the 6 Fr treatment catheter with the ultrasound transducer clearly visible within the balloon inflated with a mixture of water and contrast.
Outcome
There was no complication at the puncture site. Abdominal or lower back pain was reported by 63% of patients following the intervention and resolved after a few days in all cases. There was no device-related serious adverse event; however, one patient had to be hospitalised due to the worsening of a pre-existing headache condition. There was no change in renal function as assessed by blood and urine analysis at all follow-up visits.
Follow-up measurements were available for eleven patients at two weeks and one month, and eight patients at two and three months. Following an acute decrease at discharge (–43/–34 mmHg) caused by the intervention itself as well as the subsequent hospitalisation, the office blood pressure stabilised at two weeks (–26/–13 mmHg) and then showed a pronounced and sustained decrease at one, two, and three months when compared to baseline (–30/–15 mmHg, –32/–14 mmHg, and –36/–17 mmHg, respectively). All changes in office blood pressure were found to be statistically significant (Figure 3). This trend was confirmed by the home blood pressure measurements, which revealed a pronounced and sustained decrease at two weeks, one, two, and three months when compared to baseline (–13/–8 mmHg, –20/–11 mmHg, –19/–10 mmHg, and –22/–12 mmHg, respectively). All changes in home blood pressure were found to be statistically significant with the exception of the decrease in diastolic blood pressure at three months (Figure 4).
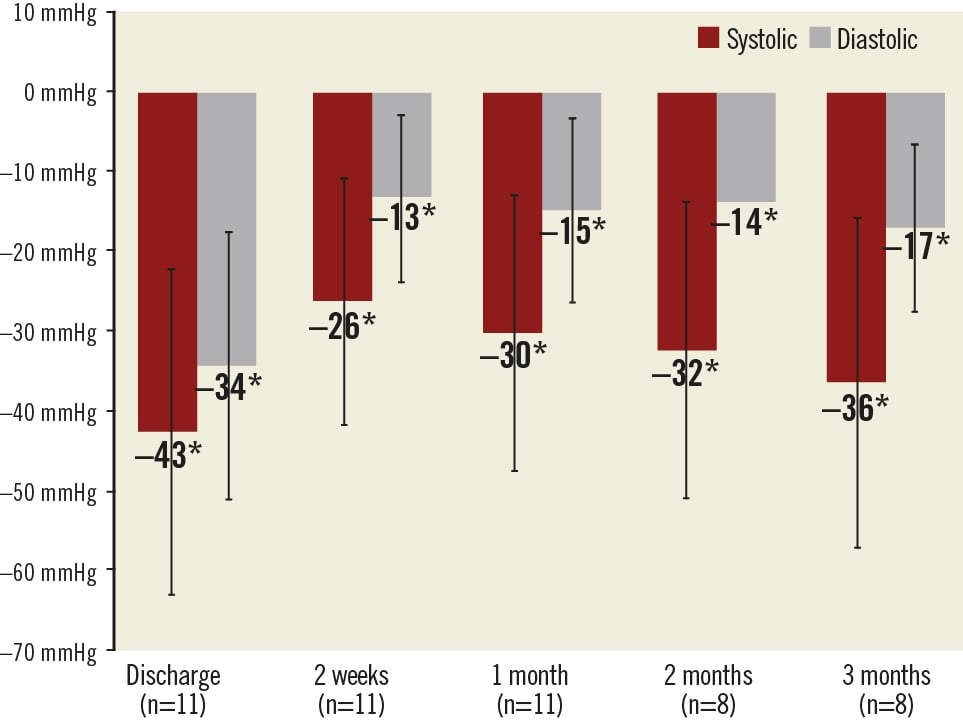
Figure 3. Changes in office blood pressure with 95% confidence intervals. The asterisks * show statistically significant (p<0.05) changes from baseline.
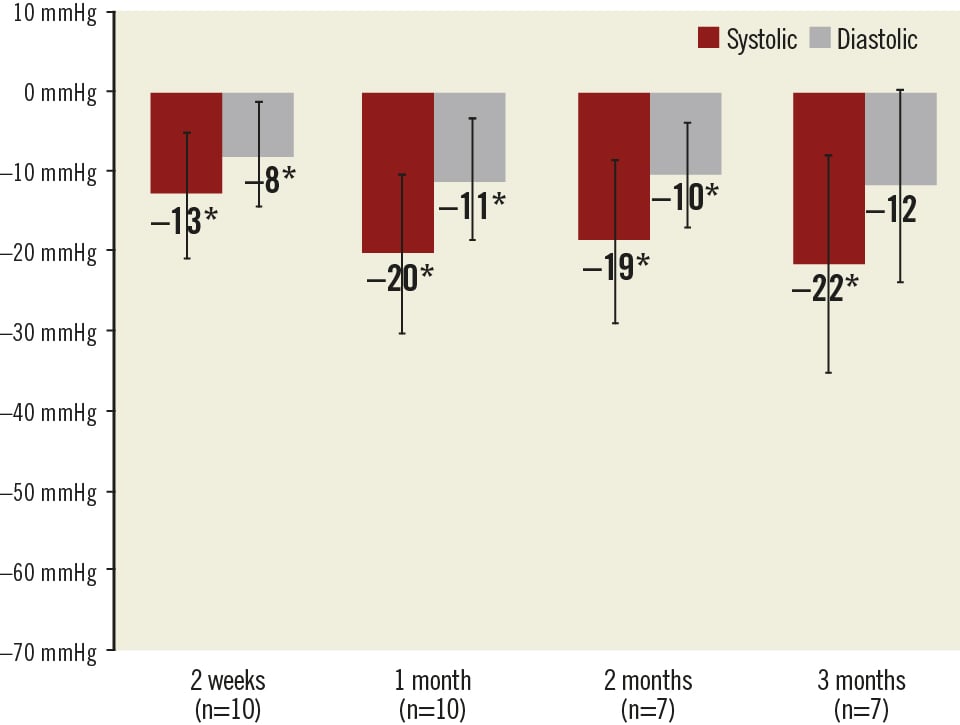
Figure 4. Changes in home blood pressure with 95% confidence intervals. The asterisks * show statistically significant (p<0.05) changes from baseline.
The clinical investigation plan called for patients to continue taking their antihypertensive medications during the course of the REDUCE study. However, two patients fulfilled the protocol criteria allowing for the doses and/or classes to be reduced (i.e., systolic blood pressure below 120 mmHg with symptoms of hypotension or repeated measures of systolic blood pressures below 120 mmHg) at the one month follow-up visit. The first patient (female, white, 41 years old, with an office blood pressure of 159/111 mmHg and taking five antihypertensive drugs at baseline) experienced a dramatic decrease in blood pressure following the intervention (112/70 mmHg at one month) with symptoms of hypotension; the frequency of one of her diuretics was reduced and she recovered with a controlled level of arterial pressure (office blood pressure of 126/85 mmHg at three months). The second patient (female, white, 63 years old, with an office blood pressure of 177/104 mmHg and taking four antihypertensive drugs at baseline) also experienced a dramatic decrease in blood pressure following the intervention with two consecutive measurements below 120 mmHg (114/66 mmHg at two weeks and 117/70 mmHg at one month); the dosage of β-blocker was reduced from 10 mg to 5 mg daily.
Discussion
We report here the preliminary experience of a novel approach for renal artery denervation. Eleven patients suffering from resistant hypertension as defined by the ESH-ESC guidelines were treated by transcatheter renal denervation using a novel technique based on the circumferential emission of ultrasound energy. Ultrasound renal denervation was found to ensure results comparable to those reported with radiofrequency ablation in the HTN-1 and HTN-2 trial, although with a shorter follow-up. Both office and home blood pressure measurements showed an immediate, significant and sustained decrease in blood pressure.
The ultrasound catheter was designed with the aim of allowing complete circumferential denervation in a more reliable fashion than the Symplicity™ radiofrequency ablation catheter, mainly because of the self-centring balloon surrounding the transducer. Animal studies performed prior to this first-in-man study showed circumferential renal nerve destruction with minimal endothelial damage, with a follow-up of up to six months.
In this experience, an average of 5.1 ultrasound emissions was delivered in each subject for a total duration of less than four minutes and the treatment was well tolerated by all patients. The duration of the intervention was less than that of radiofrequency renal denervation and could be further reduced in the future if one single ablation per renal artery proves to be efficient.
In conclusion, our preliminary results indicate that ultrasound renal denervation is a safe and effective treatment for resistant hypertension; however, additional patients and a longer follow-up will be performed to confirm the outcome and durability of this novel therapy.
Funding
The REDUCE study was sponsored by ReCor Medical.
Conflict of interest statement
M. Sapoval is a member of the ReCor Medical advisory board. V. Cabane and M. Iyer are consultants to ReCor Medical. The other authors have no conflict of interest to declare.
Excerpt from a reviewer
The authors report the preliminary findings of the first-in-man experience with the ReCor renal denervation catheter using circumferential ultrasound. Following the Ardian/Medtronic Simplicity system, the ReCor device was the first in line to receive CE mark in the beginning of this year. The ReCor device has the capability to overcome several of the procedural issues of the Simplicity catheter by eliminating the need to reposition the device continuously and thereby reducing procedure time and patient discomfort. Furthermore, a more complete denervation is hypothesised by using a circumferential approach. Besides the potentially beneficial properties of the device, this first experience report leaves multiple questions unanswered. Lessons from the Simplicity trial and a hand full of real-world reports learned that although mean office blood pressure reduction might be substantial, the number of non-responders is considerable and when using 24h ambulatory blood pressure monitoring, the average drop in blood-pressure was far less convincing. Although eight out of the 11 patients reached the three month endpoint, it is remarkable the authors fail to provide any insights into 24h and inter-individual blood pressure response to this potentially more powerful treatment. Finally, concerns have been raised about an increased risk of renal artery stenosis following circumferential treatment using ultrasound. We look forward to 6-month CT data addressing this issue as well as to the complete and longer-term follow-up of the population.

