Abstract
Aims: To determine if female gender is an independent predictor of in-hospital mortality after percutaneous coronary intervention (PCI) for ST segment elevation myocardial infarction (STEMI). A higher early mortality rate after STEMI has been reported in women before the widespread use of PCI in STEMI. PCI improves the prognosis of STEMI, however, the effect of PCI in women in this setting is controversial. In a large regional prospective registry, we examined the in-hospital mortality after PCI for STEMI.
Methods and results: The greater Paris area comprises 11 million inhabitants. Data from all PCIs performed in 41 centres is entered in a mandatory registry. In-hospital mortality is recorded in another hospital-based database. From 2003 to 2007, 16,760 patients were treated by PCI for STEMI <24 hours; 21.9% were women. Female patients were significantly older than men, 69.7±14.3 years versus 59.3±13.0 years (p<0.0001). The rate of diabetes mellitus and cardiogenic shock were significantly higher in women versus men, respectively 19.0% versus 15.6%, p<0.0001 and 6.7% versus 4.0%, p<0.0001. The success rate of PCI was significantly lower in women: 94.7% versus 95.9%, p=0.002. In-hospital mortality was significantly higher in women 9.8 % versus 4.3%, p<0.0001 and the impact of gender on mortality was significant only after the age of 75. By multivariate analysis, female gender is associated with higher in-hospital mortality.
Conclusions: After PCI for STEMI, female gender is still an independent predictor of in-hospital mortality.
Abbreviations
ARH: agence régionale d’hospitalisation
STEMI: ST segment elevation myocardial infarction
PCI: percutaneous coronary intervention
MI: myocardial infarction
AMI: acute myocardial infarction
OHCA: out of hospital cardiac arrest
CK: creatine kinase
CABG: coronary artery bypass surgery
Introduction
In patients presenting with ST segment elevation myocardial infarction (STEMI), primary percutaneous coronary intervention (PCI) has been shown to significantly improve survival1,2. Rescue and adjunctive PCI are effective therapies after thrombolytic therapy3,4. Studies of sex differences in mortality after myocardial infarction (MI) have consistently indicated that women have higher death rates, especially for short-term follow-ups5. Although different baseline and procedural characteristics, such as more advanced age, a higher percentage of diabetics and cardiogenic shock, late presentation and a lower rate in the use of reperfusion therapy may account for increased mortality, they are not sufficient to explain the discrepancy in outcome5,6. Furthermore, most of these studies were performed before the widespread use of PCI for STEMI. Using data from a large regional prospective registry, we examined in-hospital mortality after PCI for STEMI in women and men to determine if female gender was still an independent predictor of in-hospital mortality.
Methods
The CARDIO-ARHIF (Agence Régionale d’Hospitalisation d’Ile de France) registry was set up in 2001 by a government agency to monitor coronary diagnostic and therapeutic procedures performed in the greater Paris area7. This region comprises 11 million inhabitants and accounts for 18% of the French population. A total of 41 hospitals with facilities for both coronary angiography and angioplasty are implanted in this area. According to French guidelines, primary PCI is the preferred therapeutic option if transportation time to a catheterisation laboratory is less than 45 minutes. If transportation time is longer, pre-hospital thrombolytic therapy is administered and the patient brought to an institution with a catheterisation laboratory. A coronary angiogram is recommended, either at admission in case of failed thrombolysis or within 24 hours8.
Each procedure is entered in a computer database by the physician who performed the procedure. Variables entered include: patient demographics (age, sex), diabetes, preprocedural clinical status (silent, stable or unstable angina, pre-operative assessment for cardiac valvular surgery, acute myocardial infarction [AMI], cardiogenic shock, out-of-hospital cardiac arrest [OHCA] as well as other reasons), indications of the procedure (documented ischaemia in patients with silent or stable ischaemia, ST- or T-wave changes, creatine kinase [CK] and/or troponin elevation if unstable angina, myocardial infarction, or OHCA), procedural information (number of lesions dilated, amount of stents implanted), occurrence of pre- and postprocedural complications (AMI, repeat PCI , emergency coronary artery bypass grafting [CABG], stroke, vascular complications requiring surgery, renal failure requiring dialysis, blood transfusion, death). Internal audits on 10% of cases were performed twice a year in each centre. In addition, an independent external random audit of 3% of the files was held every six months in order to ensure completeness and reliability of the data. Clinical status at discharge (dead or alive) was also recorded in another hospital-based database (PMSI database). A cross check was performed using this database to validate the deaths recorded in the CARDIO-ARHIF database.
Outcomes of interest
The aim of this study was to examine the relationship between gender and in-hospital outcome in STEMI treated by PCI within 24 hours of onset of chest pain. Data was available from 2003 to 2007. These procedures were identified in the database by screening the reasons for performing the procedure and identifying patients with the following:
– STEMI of less than 12 hours of duration or STEMI of more than 12, but less than 24 hours of duration if the operator considered emergency PCI necessary because of continuous ischaemia or complications;
– cardiogenic shock.
Patients with resuscitated OHCA were not included. STEMI is defined as a prolonged, continuous (lasting at least 20 min) chest pain despite administration of nitrates, and either ST-segment elevation ≥1 mm in standard leads or ≥2 mm in two or more contiguous precordial leads or new or presumably new left bundle branch block. Cardiogenic shock was defined, at admission, as a systolic blood pressure <90 mmHg for at least 30 minutes, or if intravenous inotropes were needed to maintain a systolic blood pressure over 90 mmHg, with end-organ hypoperfusion.
The in-hospital overall mortality rate was chosen as the primary endpoint. The secondary endpoint was the rate of in-hospital complications defined as the occurrence of one or more of the following adverse events during hospital stay: death, new or recurrent myocardial infarction, re-PCI, emergency coronary artery bypass grafting (CABG), stroke, renal failure requiring dialysis, vascular access complication requiring surgery, and/or blood transfusion. A new myocardial infarction is defined as a clinical event and/or electrocardiographic changes associated with CK rise greater than or equal to three times the upper limit of the normal value. Re-infarction is defined as the occurrence of clinical symptoms and/or development of new electrocardiographic changes associated with a new elevation of CK enzyme levels. The level of CK rise depended on the interval from the index AMI: within 48 hours the new CK level was at least twice the previous value; after 48 hours at least three times the upper limit of the normal value. In all cases, angiographic visualisation of the coronary artery reocclusion was recommended.
In-hospital death was defined as death due to any cause during the hospital stay. The exact cause of death was not registered. Deaths reported during the in-hospital stay also included those occurring after transfer to another medical institution. Clinical success was defined as at least one lesion treated successfully with a residual stenosis of less than 50% and with no in-hospital complication.
The study was designed and conducted by the authors and funded by the Agence Régionale d’Ile-de-France (ARHIF), which is a French government agency. The study was approved by the Commission Nationale D’Informatique et des Libertés (CNIL), a French government agency which supervises and approves registries.
Statistical analysis
Descriptive statistics were summarised as means for continuous variables and percentages for categorical variables. Men and women’s data were compared using the X2 test or Fisher exact test for categorical variables and Student’s t test for continuous variables. We assessed if gender was an independent risk of in-hospital mortality by performing a multivariate logistic regression adjusted for age, diabetes, cardiogenic shock, lesion and PCI procedural characteristics. Interactions were tested. Statistical analysis was performed using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC, USA). A p-value <0.05 was considered as statistical significant. Tests were two-sided.
Results
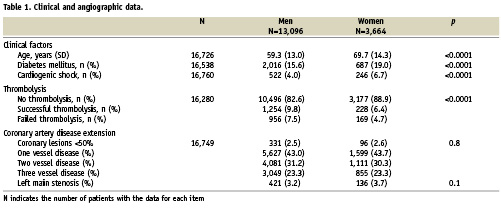
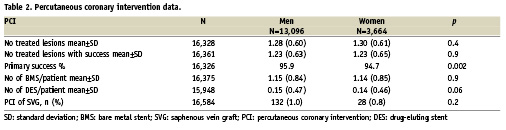
From 2003 to 2007, 16,760 patients with STEMI within 24 hours after onset of chest pain who underwent a coronary angiogram on the first day were identified in our database (3,664 [21.9%] women and 13,096 [78.1%] men). Clinical data and reperfusion strategy are displayed in Table 1. Clinical predictors of worse clinical outcome such as old age, diabetes mellitus or cardiogenic shock were significantly higher in women. Thrombolytic therapy was significantly less often administered in women. Reperfusion strategy was: primary PCI in 82.6% of men and 88.9% of women, successful thrombolysis in 9.8% of men and 6.4% of women, rescue angioplasty in 7.5% of men and 4.7% of women. There was no significant difference with respect to the extent of coronary disease and presence of left main disease in women versus men. PCI data is displayed in Table 2. The number of treated lesions was similar in women and in men as was the use of BMS or DES. However, the success rate of PCI was significantly different, 94.7% in women versus 95.7% in men, p=0.002.
A worse in-hospital outcome in women was noted with a higher mortality rate: 9.8% versus 4.3%, (chi-square test p <0.0001) (Table 3). In patients treated with thrombolysis, mortality was 3.26% in men and 7.32% in women (Crude Odds Ration [OR]: 2.34, 95% CI [1.50;3.66], p=0.0002 and adjusted OR: 1.38, [0.78;2.42], p=0.3). In patients who did not receive thrombolysis, mortality was also lower in men: 4.56% versus 10.09% (Crude OR 2.35 [2.02;2.73], p<0.0001 and adjusted OR 1.39 [1.15;1.68], p=0.0006).
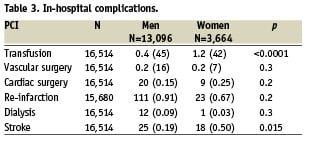
The rate of transfusion was also significantly higher (1.2% versus 0.4%, chi-square p <0.0001), but no difference was found in the rate of vascular surgery (0.2% in both groups). There was no significant difference in the rate of transfusion in patients treated by thrombolysis versus patient without thrombolysis, 0.39% versus 0.57%, p=0.2. The rate of Gp IIb-IIIa inhibitors use was 42.64% in men versus 38.18% in women (p<0.0001), and thrombolysis was 17.39% in men versus 11.11% in men (p<0.0001).
The difference in mortality between men and women was highly significant in patients aged more than 75 years: 16.1% in women versus 12.0% in men, p<0.0005. In patients aged less than 75 years of age, the mortality rate was lower in men than in women, but this difference did not reach statistical significance (Figure 1). Odds ratios (OR) for in-hospital mortality of women versus men by age intervals 20-55, 55-65, 65-75 and ≥75: 1.4 (0.82; 2.39), 1.52 (0.97; 2.39), 1.13 (0.82; 1.56), 1.41 (1.16; 1.72). There was no interaction between age intervals and gender. The OR for mortality of female STEMI patients who underwent PCI after only adjusting for age per year was 1.26 (1.07; 1.47).
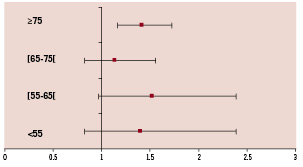
Figure 1. In-hospital mortality: unadjusted odds ratio of women versus men by age decade.
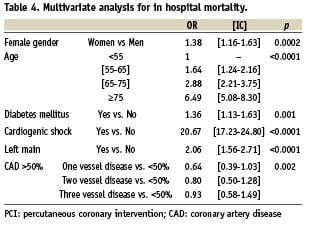
Multivariate analysis of in-hospital mortality is presented in Table 4. Predictors of in-hospital mortality were female gender, age intervals, diabetes mellitus, cardiogenic shock, coronary angioplasty failure, left main disease and failed thrombolytic therapy. The most powerful predictor of in hospital mortality was cardiogenic shock with an OR of 20.67 (17.23-24.80) followed by left main disease 2.06 (1.56-2.71), diabetes mellitus 1.36 (1.13-1.63), female gender 1.38 (1.16-1.63) and age.
In patients without cardiogenic shock (n=15,602), mortality rate was 3.5% (n=543) compared to 47.2% (n=358) in patients with cardiogenic shock (n=758), p<.0001.
Discussion
Using data from a large regional registry, we demonstrated that female gender is still an independent predictor for in-hospital mortality after myocardial infarction in the era of PCI for STEMI.
Studies of sex differences in mortality after myocardial infarction (MI) have consistently indicated that women have higher death rates, especially for short-term follow-ups5,6,9. There are a number of reasons accounting partly for this difference in outcome, namely less aggressive patient management strategies with a lower frequency of thrombolysis and PCI, less aggressive medical treatment, and more comorbidities9. Using data from the NRMI 2 registry, Vaccarino et al reported a higher use of reperfusion strategies in male patients (23% versus 16%, p<0.001) and a better in-hospital outcome, with an 11.5% mortality rate compared to 16.7% in women (p<0.001)6. Milcent et al studied data extracted from a French national health payment database10. All hospital admissions in France with a discharge diagnosis of acute myocardial infarction were extracted from the database. Women were older (75 versus 63 years of age; p<0.001) and had a higher rate of hospital mortality (14.8% versus 6.1%; p<0.0001) than men. Percutaneous coronary interventions were more frequent in men (7.4% versus 4.8%; 24.4% versus 14.2% with stent; p<0.001). Mortality adjusted for age and comorbidities was higher in women (p<0.001), with an excess adjusted absolute mortality of 1.95%. Simulation models related 0.46% of this excess to reduced use of procedures. Survival benefit related to percutaneous coronary intervention was lower among women. Similar observations were made in the Swiss registry in which female patients were older, with a higher rate of comorbidities, and underwent delayed therapeutic management with a lower rate of coronary angioplasty (odd ratio= 0.65: 0.61-0.69). In-hospital mortality was higher (10.7% versus 6.3%, p<0.001) even in patients undergoing primary PCI (4.2% versus 3.0%, p<0.018)11.
Post-analysis of randomised studies on the use of primary angioplasty have focused on sex-differences in mortality. In the PRAGUE 1 and 2 trials, the analysis of mortality in the group of patients transferred for post-thrombolysis PCI revealed a poorer outcome in the female population12. Such a difference, which was not observed in patients undergoing PCI, is partly accounted for by a longer reperfusion delay. However, randomised studies are selective, and analysis of registry data is therefore important to assess the impact of PCI for STEMI on gender differences in clinical practice.
In our study, the rate of primary PCI versus rescue or adjunctive PCI was high. In France, STEMI is managed by the pre-hospital medical emergency system with physicians in the ambulances, and guidelines are available for the management of STEMI in this setting. Decisions on reperfusion therapy and transportation are therefore swift and standardised. In the greater Paris area, a large number of cathlab facilities (41 centres) are available. As a consequence, angioplasty is the main revascularisation therapy in the majority of patients with STEMI. Interestingly, the rate of primary PCI versus rescue or adjunctive PCI was higher in female patients. Differences in mean age between men and women could account for this difference, since female patients were older and most certainly presented with contraindications to thrombolytic therapy. Primary PCI is more effective than thrombolytic therapy in reducing in-hospital mortality in STEMI1,2. Yet, despite the implementation of a pre-hospital management and triage for AMI, and the higher rate of use of primary PCI in women, in-hospital mortality was higher in female patients.
Age has been clearly shown to be a major predictive factor for mortality in STEMI, even if optimal reperfusion strategies are used6. In our study, a mean age difference of 10 years was noted. Epidemiologic data have indicated that women are relatively spared from coronary heart disease up to the age of 7513. Although the reasons for this protection are not entirely clear, oestrogen is thought to play an important role14. Women in whom coronary atherosclerosis develops before the age of 75 may be predisposed to have particularly aggressive disease, or possibly early onset, or may have more risk factors for coronary heart disease, which might override the protective effect of oestrogen. For example, diabetes has been found to negate the protective effect of female sex against coronary heart disease and death from cardiovascular disease and to be a stronger prognostic factor after myocardial infarction in women than in men15,16. In our study, women were more likely to have diabetes than their male peers and diabetes was an independent predictor of in-hospital mortality. However there was no significant interaction between sex and diabetes.
Cardiogenic shock is another factor which substantially affects the in-hospital prognosis of myocardial infarction patients especially in the first few days. In the National Registry of Myocardial Infarction 2, cardiogenic shock was more frequent in women 6.7% versus 4.0% which impacted on in-hospital outcome6. In our study, cardiogenic shock was an independent predictor of death. Some reports have suggested a lower pre-hospital STEMI mortality in men. More women are possibly admitted in cardiogenic shock which may have an impact on prognosis. However, considerable variations in the rates of pre-hospital deaths have been noted15.
Consistent with previous findings17,18, we found that older age in female patients could partially account for the higher mortality rate. Mortality rates increased according to age groups and became significant in patients aged more than 75 years of age. In contrast, using data from the National Registry for Myocardial Infarction, Vaccarino et al found that women aged less than 75 years of age had higher rates of death during hospitalisation than men of the same age. However, the use of perfusion strategy in this registry was low: the rate of female patients receiving thrombolysis was 16.2% versus 22.5% in men, with an even lower rate of thrombolysis in younger women and the rate of primary PCI is not reported6. These differences in rates of reperfusion most certainly account for the differences of mortality with our analysis: 16.7% in women and 11.5% in men versus 9.8% in women and 11.5% in men in our study. Such a difference in mortality rates could also potentially affect the results of the age range analysis. Vaccarino et al showed that in recent years, women, particularly younger ones, experienced larger improvements in in-hospital mortality after MI than men19.
Our results are similar to previous registry studies performed in patients treated by primary PCI. Cheng et al compared 30 day mortality between men and women presenting with an acute myocardial infarction of less than 12 hours and eligible for primary angioplasty20. Mortality was higher in all age ranges except between 65 and 75 years of age. However, excess mortality was greater in patients older than 75 years (30.6% in women versus 13.2% in men).
In the New York registry including 11,162 men and 2,561 women (aged less than 50) who underwent initial primary PCI for STEMI, female gender was found to be an independent predictor of in-hospital mortality despite comparable PCI success in men and women21. In our study, we found a higher rate of success for PCI in men. However, PCI failure was not a predictive factor of mortality.
In our analysis, the rate of vascular complications was low and similar in both groups. The rate of vascular surgery was higher in women; however, the numbers are small. In contrast, in the New York registry, vascular complications were significantly more frequent in women (0.82% versus 0.24%, p<0.0001). Higher rates of vascular complications in women have been consistently observed after PCI, and even coronary angiography in stable patients22. A higher rate of bleeding complications after primary PCI for STEMI was also noted by Cheng et al, (7.6% in women vs. 3.5% in men)20. Bleeding complications have been associated with higher mortality rates in studies assessing anticoagulant and antiplatelet therapies23,24. In our study, the lower rate of bleeding complications was probably related to the frequent use of the transradial approach. Although information on the type of vascular approach was not entered in the database, registries on PCI in France indicate that the radial approach is used in more than 50% of cases25.
Limitations
Partial clinical and angiographic data was entered in the base. This approach was chosen to facilitate external audits and obtain a high rate of patients with complete and reliable data, mortality was obtained from another independent hospital-based source, further improving the quality of the data. Follow-up was limited to the hospital stay. Analysis of subgroups in registries is limited by the observational nature of the analysis. However, the large number of patients included in our registry limits this potential bias. Finally, our registry is limited to patients who were catheterised within 24 hours of an acute myocardial infarction and no data is available on patients who were not investigated.
In conclusion, a registry based analysis in patients receiving optimal reperfusion therapy with PCI for STEMI of less than 24 hours demonstrated that female gender is associated with higher in-hospital mortality. There is a clear need for further studies to explain this difference, so that gender inequities in clinical care can be eliminated.
Acknowledgements
We wish to thank the steering committee: Jean-Pierre Tresca, Mireille Mapouata and Xavier Mouranche for their contribution to the Cardio-ARHIF registry.
Appendix
Principal investigators (Physicians and DIM) of the CARDIO-ARHIF registry:
Dr Anconina, Dr Sebagh and Dr Renouf, Hôpital Privé de l’Ouest Parisien, Trappes; Dr Aptecar and Dr Grandcoin, Clinique Les Fontaines, Melun; Dr Benamer and Dr Deoliveira, Hôpital Européen de Paris - La Roseraie, Aubervilliers; Dr Beverelli, Dr Makowski and Dr Bucquoit, Clinique Ambroise-Paré, Neuilly-sur-Seine; Dr Brami, Dr Jacquemet and Dr Gazi, CMC de l’Europe, Le Port Marly; Dr Brenot and Dr Herman, Centre Cardiologique d’Evecquemont, Evecquemont; Dr Cattan and Dr Lesgourgues, CHI Le Raincy-Montfermeil, Montfermeil; Dr Dibie, Dr Baudiment and Dr Bonnet, Institut Mutualiste Montsouris, Paris; Dr Drieu, Dr Leminou and Dr Gueyouche, Clinique Turin, Paris; Dr Drobinski (deceased), Pr Metzger, Dr Le Feuvre, Dr Rufat and Pr Baudelou, Hôpital Pitié-Salpétrière, Dr Durand, Dr Cador and Dr Housni, Clinique Bizet, Paris; Dr Elhadad and Dr Echard, CH de Lagny, Lagny; Dr Fourchard and Dr Menguy, CH André Grégoire, Montreuil; Dr Francoual and Dr Leclerc, CMC Foch, Suresnes; Dr Ghannem, Dr Godard and Dr Desrues, CH de Gonesse, Gonesse; Dr Guerin (deceased), Dr Favereau, Dr Dambrin, Dr Baget, Dr Debris, Dr de Livron, CMC Parly 2; Dr Guillard, Dr Funck and Dr Decup, CH de Pontoise, Pontoise; Dr Jais, Dr Tarragano and Dr Richard, Hôpital Américain de Paris, Neuilly; Dr Joseph, Dr El Mahmoud and Dr Aegerter, Hôpital Ambroise-Paré; Dr Juliard and Dr Lê-Leplat, Hôpital Bichat, Paris; Dr Karrillon, Dr Akesbi and Dr Peyron Fourcade, CH Simone Veil, Eaubonne; Dr Lancelin, Dr Caussin and Dr Vallet, CMC Marie Lannelongue, Le Plessis-Robinson; Dr Ledru, Dr Blanchard, Pr Lafont, Dr Heudes and Pr Chatellier, Hôpital Européen Georges-Pompidou, Paris; Dr Lefèvre, Dr Morice, Dr Dumas, Dr Vollaire and Dr Gedin, Institut Hospitalier Jacques Cartier; Dr Livarek, Dr Georges and Dr Vinas, CH de Versailles, Le Chesnay; Dr Loubeyre, Dr Garot, Dr Vollaire and Dr Servignes, Hôpital Privé Claude Galien - ICPS; Dr Marchand and Dr Patte, CHI Poissy-Saint Germain en Laye, Poissy; Dr Montely and Dr Benaceur, CH Robert Ballanger, Aulnay-sous-Bois; Dr Pagny and Dr Pioger, Clinique Alleray Labrouste, Paris; Dr Pernes and Dr Gedin, Hôpital Privé d’Antony, Antony; Dr Pezzano, Dr Toussaint and Dr Lairy, CHG de Corbeil-Essonnes, Corbeil-Essonnes; Dr Ronteix, Dr Faivre and Dr Buzzy, CH de Mantes la Jolie, Mantes la Jolie; Dr Royer, Dr Guyon and Dr Carradot, Clinique Cardiologique du Nord, Saint-Denis; Dr Saudemont, Dr Carreres and Dr Chevalier, CH Victor-Dupouy, Argenteuil; Dr Wyart, Dr Dumant and Dr Casciani, CHI de Villeneuve-St-Georges, Villeneuve-St-Georges; Pr Azancot, Dr Henry, Dr Lebrun, Dr Brechat and Dr Segouin, Hôpital Lariboisière, Paris; Pr Michel, Dr Stratiev and Dr Lukacs, Hôpital Tenon, Paris; Pr Monsegu, Dr Schiano and Dr Romary, Hôpital du Val de Grâce, Paris; Pr Slama and Dr Civadier, Hôpital Antoine-Béclère, Clamart; Pr Spaulding, Dr DREAU and Dr Frenkiel, Hôpital Cochin, Paris; Pr Teiger, Dr Champagne and Dr Hemery, Hôpital Henri Mondor, Créteil.

