Abstract
Aims: Cilostazol has been associated with reduction in restenosis in patients undergoing coronary and peripheral arterial angioplasty. Our objective was to evaluate the impact of cilostazol on restenosis in patients undergoing contemporary PCI with bare metal (BMS) or drug eluting stents (DES) and treated with aspirin and thienopyridine.
Methods and results: Ten randomised trials (n=2,809 patients) comparing triple antiplatelet therapy (aspirin, thienopyridine and cilostazol) with standard dual antiplatelet therapy were included. Summary risk ratios for restenosis, late loss, target lesion revascularisation (TLR) and target vessel revascularisation (TVR) were calculated using fixed-effects models. Cilostazol was associated with a significant reduction in late loss in BMS (mean difference 0.24 mm, 95% CI 0.15-0.33, p<0.001) and DES groups (mean difference 0.12 mm, 95% CI 0.07-0.18, p<0.001). Cilostazol therapy was associated with a significant reduction in angiographic restenosis (Odds ratio [OR] 0.52, 95% CI 0.41- 0.66, p<0.001) with consistent benefits in patients treated with BMS (OR 0.49, 95% CI 0.35-0.70, p<0.001) or DES (OR 0.54, 95% CI 0.38-0.76, p=0.001). Addition of cilostazol to dual antiplatelet therapy was associated with a significant reduction in TLR (OR 0.38, 95% CI 0.25-0.58, p<0.001), with no difference in subacute stent thrombosis (OR 1.91, 95% CI 0.33-11.08, p=0.47), or major bleeding (OR 0.87, 95% CI 0.44-1.74, P=0.69) but with an increased risk of skin rash (OR 3.67, 95% CI 1.86-7.24, p<0.001).
Conclusions: Cilostazol in addition to dual antiplatelet therapy is associated with a reduction in angiographic restenosis in patients undergoing stent based PCI. This inexpensive drug may be particularly beneficial in patients who are at high risk of restenosis and it should undergo further evaluation in large, definitive randomised controlled trials.
Introduction
Restenosis is a significant problem after percutaneous coronary intervention (PCI) and results in reduced quality of life and increased costs1. Although drug-eluting stents (DES) have decreased the incidence of restenosis, they have not been able to eliminate it2,3. Cilostazol, an inexpensive antiplatelet agent, has been demonstrated to reduce neointimal hyperplasia and smooth muscle proliferation after BMS or DES implantation1,4 and post endovascular therapy in patients with peripheral vascular disease5. A recent meta-analysis6 evaluating the impact of cilostazol on restenosis after PCI combined trials with variable modes of interventions (directional coronary atherectomy, angioplasty, bare metal stents) where the mechanism of restenosis itself is variable. Further, the analyses did not include patients treated with DES and its relevance to contemporary practice is uncertain.
The purpose of this meta-analysis was to systematically evaluate the impact of cilostazol on restenosis using currently available data, comparing triple therapy containing aspirin, thienopyridine and cilostazol (triple antiplatelet therapy) with dual therapy alone in CAD patients undergoing PCI with contemporary DES or BMS.
Methods
We performed a computerised search to identify relevant articles from 1996 through November 2008 using MEDLINE (National Library of Medicine, Bethesda, MD, USA), Google Scholar (Google Inc., Mountain View, CA, USA), Embase, ISI Web of Knowledge, Current Contents, International Pharmaceutical Abstracts databases, and the Cochrane Central Register of Controlled Trials. For MEDLINE we used the modified Robinson and Dickersin strategy as described by Biondi Zoccai et al7 using the keywords “cilostazol”, “PCI” , “triple antiplatelet therapy” and “restenosis”.
Abstract lists from the 2005 through 2008 scientific meetings of the American Heart Association, the American College of Cardiology, the European Society of Cardiology, published review articles, editorials, and internet-based sources of information (www.cardiosource.com, www.tctmd.com, www.crtonline.org, www.theheart.org, www.medscape.com ) were reviewed.
A study was included if it randomised patients undergoing PCI to triple antiplatelet therapy (cilostazol + aspirin + thienopyridine) or conventional therapy (aspirin + thienopyridine) in a randomised fashion and who had follow-up data on clinical or angiographic restenosis. Data was independently abstracted by two reviewers (UT, PM) and disagreements were resolved by consensus. Reviewers were not blinded to study authors or outcomes. Baseline demographic, clinical and angiographic characteristics including mean age of patients enrolled, percent of male participants, patients with diabetes mellitus, left ventricular ejection fraction (LVEF), use of platelet glycoprotein IIb/IIIa receptor inhibitor, type of stent used, were recorded for each study. We also assessed trial quality by evaluating specific elements of study design (i.e. concealment of allocation during randomisation, intention to treat analysis and blinded assessment of outcome measures), but did not use a quality score given the limitations inherent to such an approach8. Given the small number of trials available, we did not exclude any trial based on study characteristics. Attempts were also made to retrieve the data from the original source in unpublished studies.
Endpoints
Six-month angiographic endpoints included binary restenosis rate and in-segment late loss (millimetres). Binary restenosis was defined as a diameter stenosis >50%. Late loss was defined as minimum lumen diameter (MLD) immediately post PCI minus MLD at six months follow-up.
Clinical endpoints of interest included death, target lesion revascularisation (TLR), and bleeding at six to eight months follow-up. Death was defined as mortality from any cause. Target lesion revascularisation (TLR) was defined as any repeat percutaneous intervention of the target lesion or bypass surgery of the target lesion.
The safety endpoint included major bleeding as per individual study definition. Subacute stent thrombosis was defined as angiographically confirmed occlusion of the stented segment or, in the absence of angiography, the occurrence of MI or cardiac death within 30 days after the index hospitalisation.
Statistical analysis
From each trial, results were organised into a two-by-two table to permit calculation of effect sizes for triple antiplatelet therapy in comparison with dual therapy in regards to each outcome. Data on the results were collected on an “intention-to-treat” basis. When the outcome did not occur in either group, we were unable to calculate effect sizes due to the empty cells and data were excluded from that particular trial. We used fixed-effects and random-effects models to produce across-study summary odds ratios (OR) with 95% confidence intervals. As there was no heterogeneity, fixed effects results are preferentially reported. All p values were 2-tailed, with statistical significance set at 0.05. To assess the effect of individual studies on the summary estimate of effect, we did an influence analysis, in which the pooled estimates were recalculated omitting one study at a time.
Heterogeneity was assessed by means of Cochrane Q heterogeneity test and considered significant when p value was <0.109. Publication bias was assessed by plotting a funnel plot and calculating the rank order correlation10 and Eggers test of intercept11. We also calculated fail-safe N, using Rosenberg’s and Orwin’s method12-14. To provide a more clinically relevant comparison of the two regimens, risk differences were calculated for TLR and skin rash. Corresponding number needed to treat (NNT) and number needed to harm (NNH) were computed as inverse of the risk difference. All analyses were performed using comprehensive meta-analysis software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
A total of 282 citations published between January 1996 and December 2008 were screened. Studies, which compared the two strategies without randomisation15-18 were excluded. Randomised control trials comparing cilostazol versus aspirin alone19,20 or cilostazol versus thienopyridine with background aspirin therapy21-31 were excluded. Angioplasty32-35 or DCA36 trials were also excluded. Of the remaining 16 trials, six trials were excluded since they did not provide follow-up angiographic and/or mortality data37-41. Our meta-analysis thus included ten trials that randomised patients undergoing stent based PCI to triple antiplatelet therapy versus conventional antiplatelet therapy. Of these, seven trials had been published in peer-reviewed journals1,4,42-46 while three trials had been presented at scientific meetings47-49. Our final analysis thus included 10 randomised controlled trials that enrolled total of 2,809 patients (Figure 1).
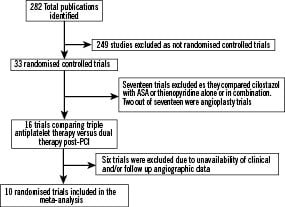
Figure 1. Flow diagram depicting the selection of studies included in the meta-analysis.
The characteristics of included trials and study populations are shown in Table 1.
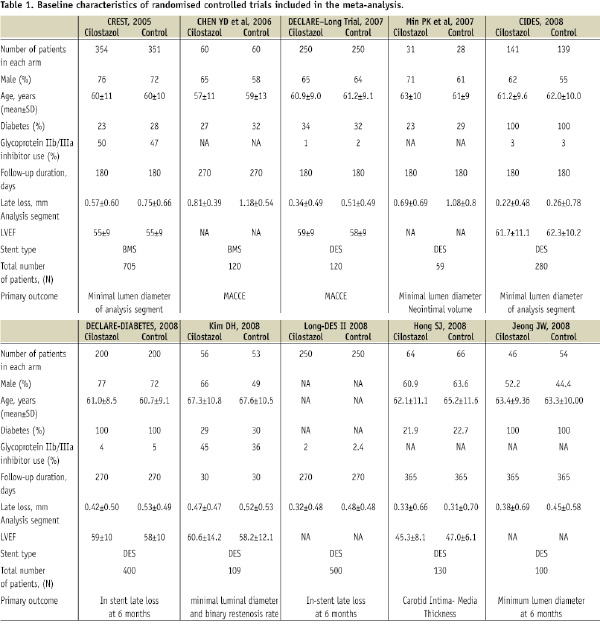
Seven trials used DES while BMS were used in three trials. The raw clinical and angiographic events in each arm across the trials are listed in Table 2.
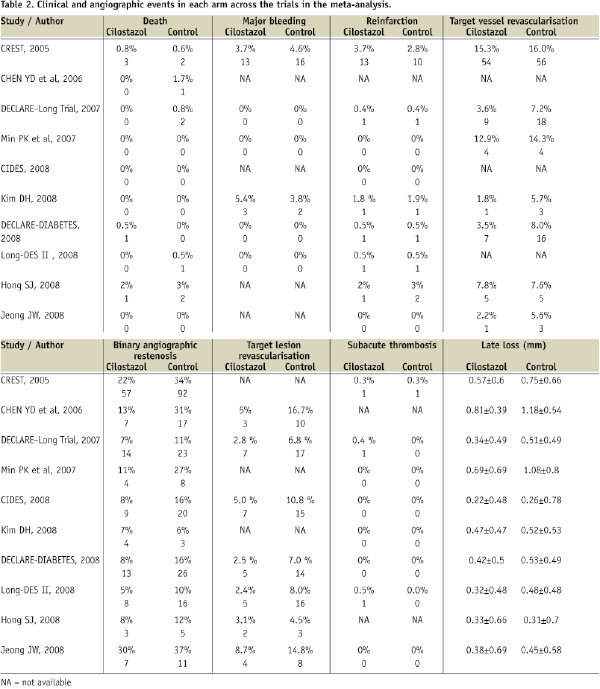
The average follow-up in these trials ranged from six to nine months.
Clinical endpoints
Data on six to nine month mortality were available in 2,809 patients (100%). Mortality in the trials ranged from 0 to 2% in the triple antiplatelet therapy arm and 0 to 3% in the standard antiplatelet therapy arm. Death occurred in five patients in the cilostazol group and in eight patients in the dual antiplatelet group. There was no significant difference in the incidence of death (OR 0.73, 95% CI 0.25-2.12, P=0.56) on follow-up between cilostazol and standard antiplatelet therapy.
Subgroup analysis did not show any significant difference in mortality between the two antiplatelet regimens in patients treated with BMS (OR 1.04, 95% CI 0.22- 5.0, P=0.96), or DES (OR 0.54, 95% CI 0.13-2.31, P=0.40).
Reinfarction
Data on reinfarction were available in 2,689 patients (95.7%) from nine trials. The incidence of reinfarction ranged from the 0 to 3.7% in the cilostazol arm and 0 to 3% in the standard antiplatelet therapy arm. Overall, reinfarction occurred in 18 patients in the triple antiplatelet therapy group and 16 patients in the dual therapy group (OR 1.12, 95% CI 0.57-2.24, P=0.74). There was no difference in infarction rates between patients treated with triple antiplatelet therapy compared with those treated with dual antiplatelet therapy among patients treated with BMS (OR 1.30, 95% CI 0.56-3.01, P=0.54) or DES (OR 0.83, 95% CI 0.25-2.78, P=0.77).
6-month angiographic endpoints
IN-SEGMENT LATE LOSS
Data on late loss at 6-month follow-up angiography were available in 2,253 (80.2%) patients from ten trials. The mean late loss ranged from 0.22 mm to 0.81 mm in the cilostazol group and from 0.26 mm to 1.18 mm in the standard therapy group. There was a significant reduction in late loss in patients on cilostazol therapy post-PCI compared to dual therapy (mean difference 0.15 mm, 95% CI 0.11-0.20, p<0.001).
The reduction in late loss associated with cilostazol was evident in association with BMS (mean difference 0.24 mm, 95% CI 0.15-0.33, p<0.001) as well as in patients treated with DES (mean difference 0.12 mm, 95% CI 0.07-0.18, p<0.001 Figure 2).
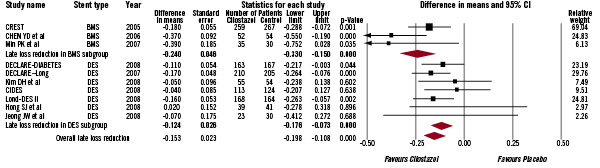
Figure 2. The Forest plot of mean difference of late loss. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars=95% CI.
BINARY ANGIOGRAPHIC RESTENOSIS
Data on binary angiographic restenosis on 6-month follow-up were available in 2,253 (80.2%) patients from ten trials. The rate of angiographic restenosis observed in the trials ranged from 5 to 30% in the cilostazol arm and 6 to 37% in the dual therapy arm. Overall, binary angiographic restenosis occurred in 126 patients in the triple antiplatelet therapy group and 221 patients in the dual therapy group. Triple antiplatelet therapy was associated with a significant reduction in the risk of restenosis at six months compared to patients on dual antiplatelet therapy (OR 0.52, 95% CI 0.41-0.66, P<0.001 Figure 3).
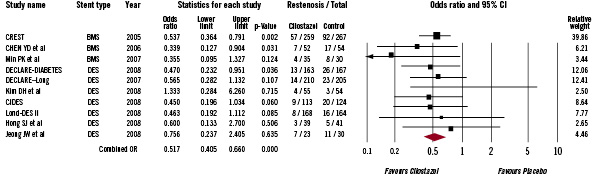
Figure 3. The Forest plot of odds ratios of binary angiographic restenosis. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars=95% CI.
Triple antiplatelet therapy with cilostazol showed a significant reduction in risk of restenosis irrespective of the type of stent implantation (BMS group, OR 0.49, 95% CI 0.35-0.70, P<0.001; DES group, OR 0.54, 95% CI 0.38-0.76, P=0.001 Figure 4).
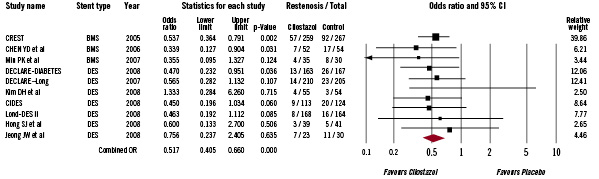
Figure 4. The Forest plot of odds ratios of binary angiographic restenosis stratified by type of stent. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars=95% CI.
Sensitivity analysis in which the pooled estimates were recalculated omitting one study at a time, did not alter any of the results.
TARGET LESION REVASCULARISATION (TLR)
Data on TLR were available in 1,936 patients (68.9%) from seven trials. The incidence of TLR ranged from the 2.4 to 8.7% in the cilostazol arm and 4.5 to 16.7% in the standard therapy arm. Overall, TLR occurred in 33 patients in the triple antiplatelet therapy group and 83 patients in the dual antiplatelet therapy group. There was a significant reduction in TLR with cilostazol therapy as compared with dual therapy alone (OR 0.38, 95% CI 0.25-0.58, p<0.001).
The reduction in TLR was more robust in patients receiving DES (OR 0.40, 95% CI 0.26-0.62, p<0.001) and only of borderline significance in those treated with BMS (OR 0.26, 95% CI 0.07- 1.01, p=0.05 Figure 5).
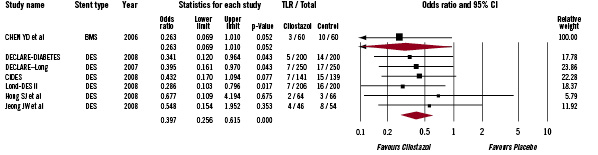
Figure 5. The Forest plot of odds ratios of target lesion revascularisation stratified by type of stent. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars=95% CI.
The number needed to treat (NNT) was 21 (95% CI 14-38) in the overall cohort while it was 22 (95% CI 15-42) in DES patients. Similarly, among patients on whom data on TVR were available, use of cilostazol was associated with a lower odds of TVR among patients treated with DES (OR 0.50, 95% CI 0.30-0.85, P=0.01), but was not significant in those treated with BMS implantation (OR 0.94, 95% CI 0.64-1.40, P=0.77).
SUBACUTE STENT THROMBOSIS (SAT)
Data on SAT were available in 2,516 patients (89.5%) from eight trials. The incidence of SAT ranged from the 0 to 0.5% in the cilostazol arm and 0 to 0.3% in the standard therapy arm. Overall, SAT occurred in three patients in the triple antiplatelet therapy group and one patient in the dual antiplatelet therapy group. There was no difference in SAT between patients treated with cilostazol compared with those treated with standard therapy (OR 1.91, 95% CI 0.33-11.08, P=0.47).
SAFETY ENDPOINTS
Data on major bleeding were available in 2,179 patients (77.6%). Major bleeding in the trials ranged from 0 to 5.4% in the triple antiplatelet therapy arm and 0 to 4.6% in the standard therapy arm. In the pooled estimate, major bleeding occurred in 16 patients in the cilostazol group and 18 patients in the dual therapy group. There was no difference in major bleeding between patients treated with triple antiplatelet therapy compared with those treated with standard therapy (OR 0.87, 95% CI 0.44-1.74, P=0.69).
Adverse drug effects
SKIN RASH
Data on skin rash during follow-up were available in 1,306 patients (46.4%). The incidence of skin rash in the trials ranged from 4.8 to 7.5% in the triple antiplatelet therapy arm and 1.2 to 2.5% in the standard antiplatelet therapy arm. Patients on cilostazol were significantly more likely to develop a skin rash compared with those on standard dual anti-platelet therapy (39 patients in the triple antiplatelet therapy group compared with 11 patients in the standard therapy group, OR 3.67, 95% CI 1.86-7.24, p<0.001 Figure 6).
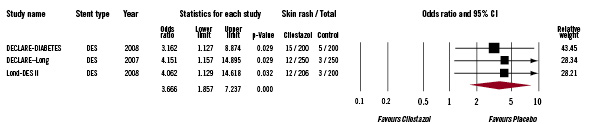
Figure 6. The Forest plot of odds ratios of skin rash. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars=95% CI.
The number needed to harm (NNH) for a rash for patients treated with cilostazol was 25 (95% CI 16-48).
Publication bias
There was no evidence of publication bias for the various endpoints on formal testing. The calculated fail-safe N for a binary angiographic restenosis was 49. This means that 49 ‘null’ studies would be needed in order for the combined 2-tailed p-value to exceed 0.05. Calculation of Orwin’s fail-safe N, assuming a mean odds ratio of 1.5 in the missing studies was 17, suggesting that 17 studies with a mean odds ratio of 1.5 would be needed to nullify the results.
Discussion
Our data suggests that therapy with cilostazol, over a background of aspirin and clopidogrel, is associated with decreased late loss with a resultant decrease in rates of angiographic restenosis and a decreased need for TLR, without a significant difference in the risk of bleeding and stent thrombosis. The clinical impact of cilostazol therapy appears to be particularly evident in patients treated with DES as shown by a 50% reduction in TLR.
Several mechanisms have been proposed to explain the antiproliferative effects of cilostazol. It is a potent phosphodiesterase III (PDE III) inhibitor preventing the hydrolysis of cAMP in platelets and vascular smooth muscle cells. The increase in the intracellular levels of cAMP results in up regulation of anti-oncogenes p53 and p21 and increased production of hepatocyte growth factor. This results in apoptosis of the smooth muscle cell and reduces its proliferation and migration. The hepatocyte growth factor accelerates re-endothelialisation and endothelial stabilisation improving endothelial function50.
Cilostazol mediates an anti-inflammatory effect by inhibiting leukocyte integrin (Mac-1) either directly or indirectly by inhibition of expression of P-selectin on platelets and subsequent P-selectin-PSGL-1 (P-selectin glycoprotein ligand-1) signalling31. Increased Mac-1 expression and activation during coronary interventions has been linked to neointimal thickening and restenosis51.
A recent meta-analysis of 23 studies showed a potential benefit of cilostazol in reducing angiographic restenosis and improved clinical outcomes but acknowledged potential bias due to disproportionate inclusion of positive small studies. The analysis included trials comparing cilostazol against variable background therapy (aspirin and/or thienopyridine) and a host of interventional strategies. The included trials encompassed angioplasty and DCA along with early generation stent trials where the mechanism of restenosis may have been different. Further, due to paucity of DES trials in the analysis, the role of triple antiplatelet therapy in patients receiving contemporary PCI could not be completely evaluated.
In current practice, aspirin plus thienopyridine remains a potent background therapy and has decreased the risk of stent thrombosis significantly. Thus, the beneficial role of cilostazol on restenosis and stent thrombosis can only be studied by comparing triple antiplatelet therapy against this potent dual therapy.
The natural history of stent restenosis has only recently been unravelled, and contrary to earlier assumptions of a benign entity, an increased risk of myocardial infarction and late mortality in association with restenosis has been demonstrated52-54. While DES has significantly ameliorated the problem, restenosis remains a significant clinical problem in a small, but significant, subpopulation. Of particular importance, the impact of cilostazol was more significant in patients treated with DES compared with BMS, even though the absolute difference in late loss was greater in patients treated with BMS. This may relate to the sigmoid association between late loss and TLR, where the small decrease in late loss in DES patients may be particularly powerful in patients on the steep part of the curve, while the greater absolute decrease in BMS may be less clinically meaningful on the relatively flat part of the curve.
As an inexpensive generic drug with a 6-month cost of less than 180 American dollars, cilostazol may be one of the most cost-effective strategies to reduce the risk of restenosis. This may be particularly relevant for patients at the highest risk of restenosis, such as those with diabetes and long lesions, where the most dramatic reductions in TLR have been reported with triple antiplatelet therapy. A formal cost-effectiveness analysis would be valuable to assess the financial burden from the addition of cilostazol to existing dual antiplatelet therapy.
The reduction in restenosis does not appear to carry with it an increased risk of bleeding, although the incidence of skin rash was rather high in these trials (5%-8%). The NNT for TLR and NNH for skin rash were fairly similar such that for every 1,000 patients treated with triple antiplatelet therapy, TLR was prevented in 47 patients with the occurrence of skin rash in 41 patients. Further research is needed to develop a bio-designer drug that avoids the rash associated with cilostazol while retaining its beneficial anti-restenotic effects.
Limitations
The limitations of our meta-analysis are those inherent to all meta-analyses, and they include publication bias (although tested non-significant in our study) as well as the difficulties in comparing the results because of different study populations, study designs and reporting methods, not to mention the absence of individual patient data which prohibits adjustment for confounding factors55. The study population in some trials was composed exclusively of diabetic patients known to be at a high risk for restenosis. Similarly, some of the studies included patients undergoing PCI irrespective of their clinical status (“all comers”) while others are restricted to patients with myocardial infarction. The restenosis rates in some of the included studies were higher than what has been reported in prior published studies of DES, and may suggest an enrolment of particularly high-risk patients. This difference in the baseline risk of restenosis in study populations may be especially relevant for the cost effectiveness and maximal anti-restenotic benefit from triple therapy which is likely to be observed in the highest-risk populations (diabetics, long lesions). Data on clinical endpoints like TLR and reinfarction were not available in some trials, and data on skin rash was available in only three trials. Data on the number of patients who discontinued the triple antiplatelet therapy were reported in only three trials. A significant discontinuation rate may suggest high incidence of side effects, and may also lead to underestimation of the true, anti-restenotic potential of the drug. Since data on incidence of discontinuation was not available in most trials, it may possibly modify the NNT and NNH values obtained. The TLR rates may have been driven by angiographic follow-up, and clinical revascularisation rates may have been lower. Most of the trials included in our analysis were conducted in Asian countries and the results may differ in other ethnic groups. The side effects of cilostazol are not restricted to skin rash and include headache, gastrointestinal disorders and palpitations. Data on these adverse effects was not available in many trials and hence could not be pooled. The results of our trial thus cannot supplant a large, adequately powered, randomised clinical trial.
Conclusion
Addition of cilostazol to conventional antiplatelet therapy in patients treated with contemporary PCI decreases angiographic restenosis with a resulting decrease in the risk of TLR. This cost-effective drug may be particularly beneficial in patients who are at high risk of restenosis, and it should undergo further evaluation in large definitive randomised controlled trials.
Acknowledgements
We would like to thank Ms. Dawn Ambs for help in formatting and submission of the manuscript.

