
Abstract
Aims: We aimed to investigate the outcomes of bioresorbable vascular scaffolds (BVS) in coronary ostial lesions. Ostial lesions represent a challenging angiographic subset, with higher event rates compared with non-ostial lesions. BVS might be associated with advantages over the long term, but their safety in this setting remains to be explored.
Methods and results: Procedural and 12-month follow-up data from consecutive patients treated with BVS for lesions located at the ostium of the right (RCA), left anterior (LAD) or circumflex (LCX) coronary in 11 European centres were collected. The primary device-oriented endpoint was defined as a combination of cardiovascular death, target vessel myocardial infarction or target lesion revascularisation. The database included a total of 1,549 lesions in 1,304 patients with a mean age of 62±11years. There were 90 ostial lesions (5.8%) in 84 patients (6.4%) located at the ostial RCA (14; 16%), LCX (29; 32%), or LAD (47; 52%). Patients presenting with ostial lesions did not differ from the remaining cohort except for a higher incidence of prior revascularisation. Predilation was performed in 97% of the lesions (vs. 96% in non-ostial, p=0.618), post-dilation in 43% (versus 58% in the non-ostial group, p=0.008). At quantitative coronary angiography, treatment of ostial lesions was associated with higher residual stenosis (30% [23-41] vs. 26% [20-37], p=0.035), but no difference in minimum lumen diameter existed (p=0.447). Follow-up data were available at 385 [362-465] days. The 12-month Kaplan-Meier estimated rates of scaffold thrombosis were 4.9% and 2.0% (ostial and non-ostial lesion groups, respectively, log-rank p=0.005). The device-oriented composite endpoint occurred, respectively, in 12.6% and 4.6% at 12 months (log-rank p=0.001). Treatment of ostial lesions was an independent predictor of this endpoint (p=0.0025, HR 2.65 [1.41-4.97]).
Conclusions: In combination with a suboptimal implantation technique, treatment of coronary ostial lesions was an independent predictor of clinical events in a cohort of patients treated with BVS.
Abbreviations
BVS: bioresorbable vascular scaffolds
DES: drug-eluting stents
ScT: scaffold thrombosis
TLF: target lesion failure
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Bioresorbable vascular scaffolds (BVS) have recently been introduced in the treatment of coronary artery disease1,2. Following implantation, BVS undergo resorption, which allows restoration of vasomotion as well as vascular healing and positive remodelling, thus possibly preventing some of the long-term limitations associated with metallic drug-eluting stents (DES)3. While outcome data from pivotal trials of BVS are limited to relatively simple lesions, these devices are now used in progressively more complex settings of everyday practice, such as bifurcations, calcific lesions, long or thrombus-containing lesions and chronic total occlusions2,4-6.
Ostial lesions represent a rare7 but challenging angiographic subset due to the higher incidence of recoil, incomplete stent expansion and restenosis associated with the more frequent presence of calcification and fibrosis compared with non-ostial lesions8,9. Although the higher incidence of restenosis in ostial lesions has been at least partially addressed by the advent of DES, percutaneous coronary intervention of this subset of stenoses remains technically challenging and is associated with increased rates of acute and long-term complications10-13. Treatment of ostial lesions remains an independent predictor of events even with the use of second-generation DES14. Safety and outcome data of BVS in ostial lesions have not been reported so far.
We report on acute and one-year clinical outcomes following treatment with BVS for coronary ostial lesions in the multicentre GHOST-EU (Gauging coronary Healing with biOresorbable Scaffolding plaTforms in EUrope) registry2.
Methods
The GHOST-EU registry is an investigator-initiated, retrospective, multicentre registry conducted in 11 European centres in Italy, Germany, Poland, Spain and the United Kingdom. Details about the design, methods of data collection and definitions used are presented in detail in a previous publication, reporting on 30-day and six-month outcomes of the initial 1,189 patients collected2. Briefly, the registry includes consecutive patients who received at least one everolimus-eluting BVS (Absorb; Abbott Vascular, Santa Clara, CA, USA) for the treatment of coronary artery lesions between November 11, 2011, and January 29, 2014. All interventions were performed according to current standards of PCI and following CE marking. Use of post-dilation, intracoronary imaging guidance, and the choice of antithrombotic regimens were left at the discretion of the operator or centre. Quantitative coronary angiography was performed by each centre. Data on hospital admissions, procedures and outcomes were collected at each site by clinical visits or telephone contacts, and audited centrally. Patients with no available information on the lesion site along the coronary vessel (ostial versus non-ostial) represented 13% of the total cohort and were excluded from the present analysis. Ostial lesions were defined as lesions within 3 mm of the ostium of the left main, left anterior descending, circumflex or right coronary artery in the most orthogonal projection.
Definitions
Clinical outcomes of interest were based on standardised criteria14,15, and the definitions are detailed in the original GHOST-EU publication2. The primary endpoint was a device-oriented composite endpoint (DoCE) defined as the combination of cardiac death, target vessel myocardial infarction and clinically driven target lesion revascularisation (TLR). The quantitative coronary analysis was performed on-site by experienced personnel using one of the following software: Siemens Healthcare Axiom Artis VB35D110803 (Siemens Medical Solutions, Siemens AG, Forchheim, Germany); Siemens Healthcare ACOM.PC 5.01 System (Siemens Medical Solutions, Siemens AG); General Electric AW VolumeShare 6E (General Electric Inc., Fairfield, OH, USA); CASS II analysis system (Pie Medical BV, Maastricht, The Netherlands); QAngio XA (Medis BV, Leiden, The Netherlands). The minimum lumen diameter (MLD) was defined as the smallest lumen diameter in the segment of interest, and the reference vessel diameter (RVD) as the averaged diameter of the coronary assumed without atherosclerotic disease. Percent stenosis was (RVD-MLD)/RVD, and acute gain was post-procedural MLD - pre-procedural MLD.
Statistical analysis
Data are presented as counts and percentages, mean±SD or median (interquartile range). Normality was assessed by visual inspection of the Q-Q plots. Differences in proportions were tested with chi-square tests or Fisher’s exact tests, and differences in continuous variables were tested with a Student’s t-test. The Kaplan-Meier method was used to generate cumulative incidence curves for each endpoint, which were compared using the log-rank test. A univariate Cox proportional hazards regression modelling was performed to determine the independent predictors of DoCE. Variables included in the univariate analysis were age, sex, history of hypertension, diabetes, hyperlipidaemia, smoking, prior revascularisation, renal disease, left ventricular ejection fraction, acute coronary syndrome at index, intracoronary imaging, treatment of ostial lesion, post-dilation, and type of antithrombotic therapy. Variables associated at univariate analysis with a p-value <0.20 were entered in a multivariable model. Results are reported as hazard ratio (HR) with associated 95% confidence interval and p-value. The primary endpoint of the study was the association of ostial lesions with the 12-month incidence of DoCE. For this endpoint, a p-value <0.05 was chosen as significant. All other tests have to be considered as exploratory. All analyses were performed with SPSS for Windows, Version 23.0 (IBM Corp., Armonk, NY, USA) and MedCalc (MedCalc Software, Mariakerke, Belgium).
Results
PATIENT CHARACTERISTICS
A total of 2,018 BVS were implanted in 1,304 patients (1,549 lesions) and included in this analysis. Baseline patient characteristics are shown in Table 1. Ninety BVS were implanted in 84 patients (6.4% of the patients, 5.8% of the lesions) at the coronary ostium. Patients with ostial lesions had more often a history of previous revascularisation, and a trend towards higher incidence of multivessel disease. Lesions treated in the RCA were less frequently ostial.
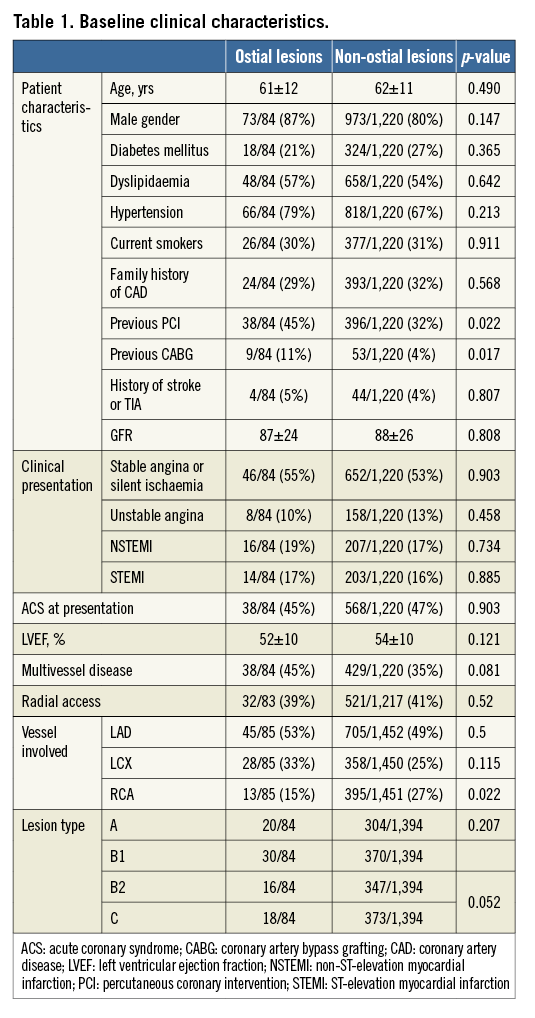
PROCEDURAL CHARACTERISTICS
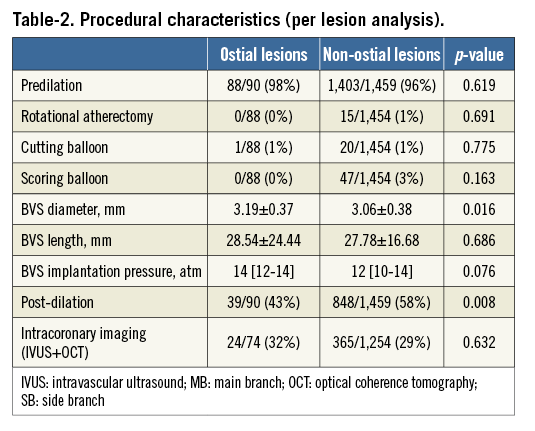
Predilation was performed in the vast majority (96%) of lesions in both groups. There was no difference between groups in the use of debulking devices. In contrast, post-dilation was more frequently performed in non-ostial lesions. The mean BVS diameter and implantation pressure were larger in the ostial lesions group (Table 2).
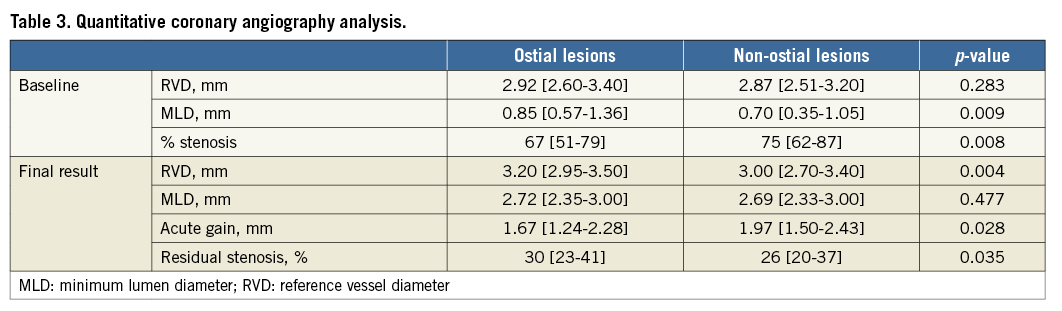
QUANTITATIVE CORONARY ANGIOGRAPHY
Quantitative coronary angiography data were available in 581 patients (Table 3). Of these, 51 patients had ostial lesions. Before intervention, the (distal) RVD was similar between groups, while the MLD was higher and the diameter stenosis lower in ostial lesions. Following PCI, RVD was larger in the ostial group, but MLD was similar between groups. As a result, acute gain was lower, and residual stenosis was larger, in ostial lesions.
CLINICAL OUTCOMES
Information on the periprocedural changes in troponin was available in 1,045 patients. A periprocedural myocardial infarction as defined by an increase in troponin levels occurred in 120 (12.3%) patients in the non-ostial group and four (5.6%) in the ostial group (p=0.136). The rate of other periprocedural complications (including acute occlusion, intraprocedural stroke or death, no reflow, asystole) was very low and not different between groups.
Clinical follow-up was available at a median of 385 (362-465) days. A 12-month follow-up was available in 1,154 patients (89% in the non-ostial and 86% in the ostial group, p=0.516). The Kaplan-Meier estimates at six and 12 months are presented in Table 4. There were 25 deaths (three in the ostial group and 22 in the non-ostial one), 49 myocardial infarctions (seven and 42), 109 target vessel revascularisations (15 and 94), 33 in-scaffold thromboses (six and 27) and 84 target lesion revascularisations (12 and 72). Figure 1 describes the 12-month Kaplan-Meier curve of DoCE in the two groups. A total of 88 DoCE were recorded (14 and 74). Of the variables listed in the statistical section, history of diabetes, hypertension, acute coronary syndrome at index and treatment of ostial lesions showed a p-value <0.20 in univariate analysis and were entered in the multivariable analysis. Treatment of ostial lesions was the strongest independent predictor of DoCE (p=0.0025, HR 2.65 [1.41-4.97]). Along with treatment of ostial lesions, the association with diabetes (p=0.011, HR 1.77 [1.14-2.75]) and acute coronary syndrome at index procedure (p<0.001, HR 2.17 [1.41-3.34]) remained significant at multivariable analysis. Other clinical, lesion and procedural variables were not associated with the event.
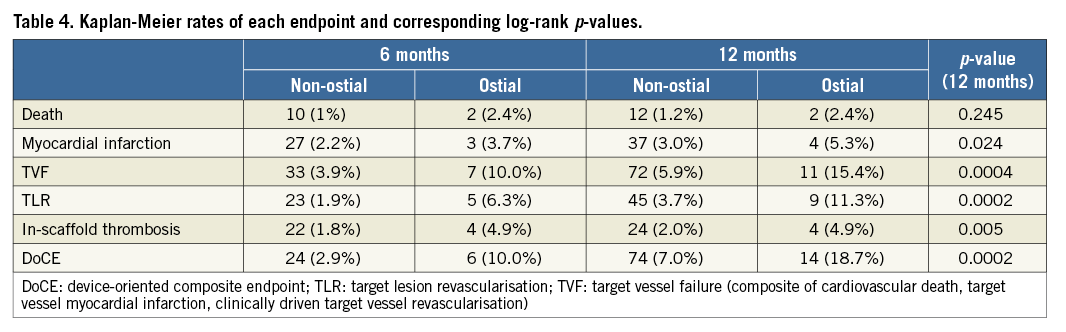
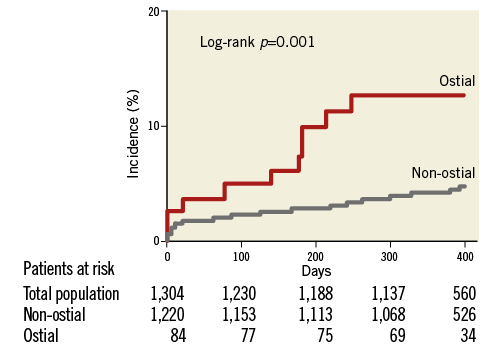
Figure 1. Kaplan-Meier curve describing the incidence of the composite endpoint.
Discussion
We report for the first time on the acute and midterm results after treatment of ostial lesions with BVS. In the present cohort, treatment of ostial lesions was an independent predictor of DoCE.
The goal of PCI for ostial lesions is to improve the distal flow while placing the stent exactly at the vessel ostium to avoid geographic miss and limit injuries of adjacent vessels, including the aorta. Correct positioning of the stent becomes particularly complex as implantation too proximal in the left main bifurcation or the aorta may result in a possible nidus for future stent thrombosis and complicates future access to the vessel. In this setting, BVS have the theoretical advantage that the resorption of the struts would remove any issue potentially associated with floating struts, thus limiting the risk of late thrombosis and simplifying further treatment in the downstream vessel over time. Two other considerations, however, further complicate the treatment of ostial lesions. First, the higher incidence of calcifications and the different organisation of smooth muscle cells in the vascular media limit stent deployment8,9. Second, coronary ostia are hinge points, particularly exposed to mechanical forces during the cardiac cycle, and devices implanted at this level are exposed to a particular type and degree of dynamic stress. Due to these factors, PCI of ostial lesions is associated with particularly high rates of acute and long-term complications, which were only partially addressed by the introduction of DES.
Principal findings
QUANTITATIVE CORONARY ANALYSIS
The mean reference vessel diameter was, as expected, larger in ostial lesions, which were also angiographically less severe as compared to non-ostial ones. Immediately after implantation, the percentage residual stenosis was larger in ostial compared to non-ostial lesions; however, this difference was exclusively driven by differences in RVD, while the minimum lumen diameter was similar between groups. Of note, this finding might have been influenced by the particular resistance to dilation of ostial lesions, but its interpretation is also complicated by differences in BVS diameter and implantation pressure and by the lower rate of post-dilation in the ostial group. It needs to be emphasised here that systematic use of post-dilation is currently recommended after BVS implantation in expert opinion papers15,16.
CLINICAL OUTCOMES
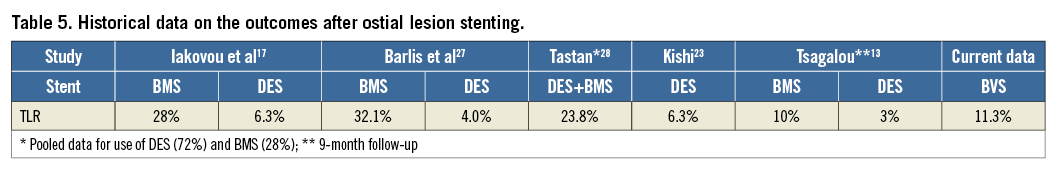
The 12-month incidence of target lesion revascularisation was higher but in the range of that previously reported for metallic DES (between 4% and 8%; Table 5), and markedly lower than that previously reported with the use of bare metal stents (in the range of 30%)13,17-20. Although the lack of a control group prevents us from reaching firm safety conclusions, the incidence of in-scaffold thrombosis was non-negligible, in keeping with our previous reports2,6,21, particularly in the ostial lesions group even when compared to previous reports of DES use in ostial lesions22,23. Finally, the incidence of DoCE was higher in ostial than in non-ostial lesions, and, similar to second-generation DES14, treatment of ostial lesions was an independent predictor of the composite endpoint. Of note, the incidence of events was comparable to that reported in previous DES studies11,22-27.
Limitations
This was a non-randomised registry, and the lack of monitoring, as well as the heterogeneity in patient selection, treatment strategies and implantation procedures across centres are important limitations. The degree of calcification and angle of the lesions was not collected in the database. Also, given the evidence suggestive of incomplete deployment of the BVS, the use of debulking devices, intracoronary imaging and post-dilation appears, in retrospect, low. The importance of these procedures is now better understood. There was no DES-treated control group, and the heterogeneity in the protocols, cohorts under study, and outcomes of previously published studies complicates any comparison11,22-29. In addition, operator preference at the time of the choice of the device (BVS versus DES) might have introduced a patient/lesion selection bias. Collection and adjudication of events was performed by each centre, but data were centrally audited for inconsistencies, and queries were issued in case of discrepancies. Furthermore, follow-up was limited to one year in the present report. Finally, there was no central angiographic core lab. Despite these limitations, the present data represent a realistic picture of the use of BVS in experienced centres across Europe, and, although limited due to the relatively low incidence of this anatomical presentation, the size of the present database allows the identification of ostial lesions as an independent predictor of outcome after BVS implantation in a real-life scenario.
Conclusions
PCI of ostial lesions with BVS was associated with an increased rate of all events, including scaffold thrombosis, compared with PCI of non-ostial lesions. In multivariable analysis, ostial lesions represented an independent predictor of clinical events. Taken together, the present data emphasise the need for particular care when treating coronary ostial lesions, including a more frequent use of post-dilation, debulking devices and intracoronary imaging. Further data are necessary before a conclusion on the safety of BVS versus DES can be made.
| Impact on daily practice Treatment of ostial lesions is complex from the technical point of view and unrewarding in terms of outcomes. We report on the outcomes following implantation of bioresorbable scaffolds for these types of lesions. As with drug-eluting stents, scaffold implantation at the level of ostial lesions remains an independent predictor of events at 12 months. |
Acknowledgements
This paper contains work included in the doctoral thesis of J. Weber.
Conflict of interest statement
T. Gori, D. Capodanno, A. Latib, M. Lesiak, S. Pyxaras, J. Mehilli, C. Di Mario, S. Brugaletta, M. Sabate, C. Naber, A. Araszkiewicz, A. Colombo, C. Tamburino, H. Nef, and T. Münzel have received speaker’s honoraria from multiple companies including Abbott Vascular. The other authors have no conflicts of interest to declare.

