Abstract
Aims: Acute stent recoil and luminal filling defects can result in a suboptimal angiographic result following stent deployment and are associated with an increased risk of adverse outcomes. We aimed to evaluate the effect of double stenting, deployment of a second stent within the first, in the treatment of these conditions and to review the literature on this procedure.
Methods and results: Thirteen cases of double stenting performed by a single operator at the Manchester Royal Infirmary over a three year period were identified and quantitative coronary angiography was performed. The indication for double stenting was acute stent recoil in eight cases, luminal filling defects in three cases and a combination of recoil and filling defects in two cases. There was a high frequency of target vessel calcification (77%) and ostial lesions (23%). Following double stenting, mean minimum lumen diameter increased significantly from 2.5 mm to 3.5 mm (p <0.001). There were no procedural complications. At mean clinical follow-up of 19 months (range six to 37 months), there were no major adverse cardiac events.
Conclusions: Double stenting can significantly improve angiographic outcome in cases of acute stent recoil and luminal filling defects, with excellent clinical results in the medium term.
Introduction
During percutaneous coronary intervention (PCI) a suboptimal angiographic appearance within the stented segment can occur due to either stent under expansion related to acute stent recoil or luminal filling defects from plaque or thrombus extrusion through the stent struts. Suboptimal expansion and residual intraluminal filling defects are both associated with adverse clinical outcomes including an increased risk of stent thrombosis and restenosis1-4. High pressure non-compliant balloon inflation within the stented segment may improve the angiographic appearance; frequently however the suboptimal appearance persists.
One strategy for treating acute stent recoil and luminal filling defects is “double stenting”, the deployment of a second stent, usually of equal diameter and equal or shorter length, within the original stent. However the results of double stenting either using bare metal stents (BMS) or drug eluting stents (DES), or a mixed approach, have not been well characterised.
We report on a series of patients treated with double stenting and review the literature on this topic.
Methods
All procedures performed by a single high-volume operator (DGF) at our institution over a three year period were retrospectively reviewed and cases of double stenting in de novo lesions were identified. The suboptimal angiographic appearance which resulted in double stenting was either a new intraluminal filling defect following stent deployment, classified as plaque or thrombus prolapse according to the operator’s interpretation of the aetiology, or acute stent recoil, defined as a persisting unacceptable residual stenosis in two orthogonal angiographic views following stent deployment and high pressure non-compliant balloon dilatation, despite evidence of adequate balloon expansion. Quantitative coronary angiography (QCA) was performed and minimum lumen diameter (MLD), reference vessel diameter (RVD) and percentage diameter stenosis were measured at baseline, following first stent deployment and high pressure balloon inflation, and at the end of the procedure following second stent deployment. Intravascular ultrasound (IVUS) was not used routinely.
Follow-up data was obtained in all cases via clinic notes and telephone questioning. Major adverse cardiac events (MACE) were defined as cardiac death, myocardial infarction (MI) and target lesion revascularisation. Repeat coronary angiography was performed only if clinically indicated or if a staged intervention was planned.
Changes in angiographic parameters were compared with the paired sample t-test, with a 2-sided p value <0.05 considered significant.
Results
Thirteen cases of double stenting were identified which represented 1.3% of the 1,033 PCI procedures performed during the three year study period. The procedural characteristics of the patients are detailed in Table 1.
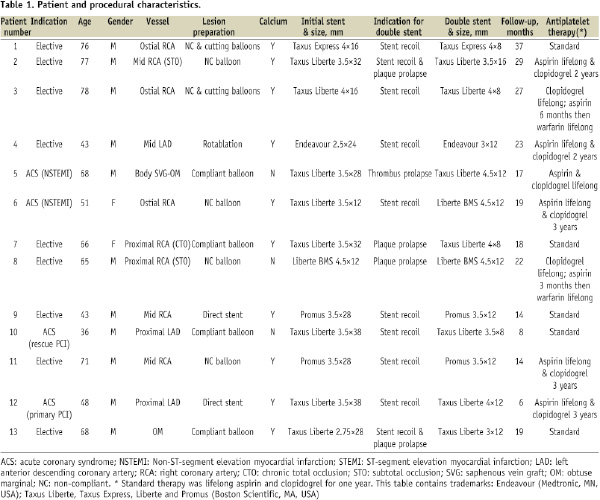
Twelve cases were performed via a radial approach. Target vessel calcification was present in ten cases and three lesions involved the coronary ostia; these three lesions all demonstrated angiographic evidence of calcification. Pre-dilatation was performed in eleven cases and required the use of non-compliant or cutting balloons in six of these. Rotablation was used in one case because of extensive calcification. In all cases double stenting was performed because of a persisting suboptimal angiographic appearance despite high pressure non-compliant balloon inflation to a minimum of 20 atmospheres following first stent deployment. Post-dilatation of the second stent was subsequently performed in all cases. The indication for double stenting was acute stent recoil in eight patients, plaque prolapse in two patients, thrombus prolapse in one patient, and a combination of stent recoil and plaque prolapse in two patients. Eleven cases were performed with two overlapping DES, one case used two overlapping BMS and one case used a combination of one BMS and one DES. The mean length of the second stent, and thus the mean length of stent overlap, was 11 mm. IVUS was used in two cases.
Quantitative coronary angiography
The QCA measurements are detailed in Table 2.
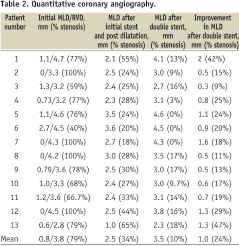
There was a significant improvement in luminal area following double stenting, with an increase in mean MLD of 1.0 mm from 2.5 mm to 3.5 mm (p <0.001) and a reduction in mean stenosis from 34% to 10% (p <0.001). For those patients with stent recoil, mean MLD was increased from 2.4 mm to 3.3 mm (p <0.001) and mean stenosis was reduced from 35% to 11% (p <0.001). Three representative cases are illustrated in Figures 1-3.
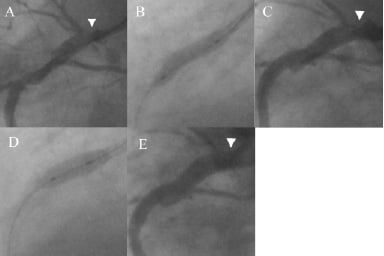
Figure 1. Coronary angiogram demonstrating double stenting for stent recoil. A) Proximal vessel following cutting balloon pre-dilatation showing severe ostial disease (arrowhead); B) Deployment of 4×16 mm Taxus Express back to ostium of RCA showing good stent expansion; C) Significant stent under expansion secondary to recoil at the ostium despite non-compliant balloon post-dilatation (arrowhead); D) Deployment of 4×8 mm Taxus Express “double stent” within the proximal stent; E) Final result shows marked increase in final lumen diameter at ostium (arrowhead).
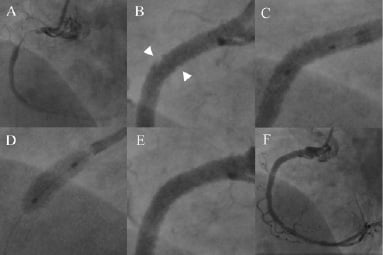
Figure 2. Coronary angiogram demonstrating double stenting for plaque prolapse. A) CTO of proximal RCA; B) Following pre-dilatation, deployment of a Taxus Liberte 3.5×32 mm stent and post-dilatation with a non-compliant balloon, there is marked plaque prolapse into the vessel lumen (arrowheads, insert); C) & D) Positioning and deployment of Taxus Liberte 4×8 mm “double stent”; E) & F) Final result.
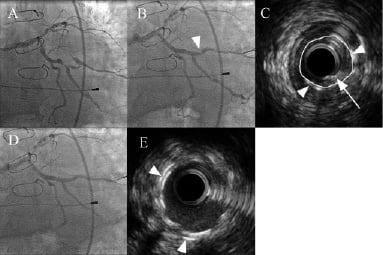
Figure 3. Coronary angiogram and IVUS demonstrating double stenting for stent recoil and plaque prolapse. A) Severe lesion in OM1; B) Following deployment of a Taxus Liberte 2.75×28 mm stent and post-dilatation there is a residual stenosis within the stented segment (arrowhead); C) IVUS of stented area showing significant stent under expansion due to recoil with sparse circumferential distribution of the stent struts (arrowheads) and evidence of an intra-stent filling defect consistent with plaque prolapse (arrow). The calculated minimum stent cross-sectional area is 1.53 mm2 and is marked in yellow; D) Final angiographic result following deployment of a Taxus Liberte 3×12 mm “double stent”; E) Final IVUS image taken at the same distance from the original stent origin as C. The double layer of stent struts can be clearly seen (arrowheads) and the cross-sectional area is significantly improved (4.77 mm2).
Follow-up
There were no procedural or in-hospital complications. Given the complex nature of these procedures the recommendations on antiplatelet therapy varied considerably (Table 1). Standard therapy in our institution for patients undergoing PCI with DES during the study period was for lifelong aspirin and clopidogrel for one year following the procedure. In this series six patients were prescribed dual antiplatelet therapy for more than one year. Two patients were in permanent atrial fibrillation and were prescribed dual antiplatelet therapy initially followed by a single antiplatelet agent and warfarin as maintenance therapy.
Clinical follow-up was performed in all patients with a mean duration of 19 months (range 6 to 37 months). During the follow-up period there were no deaths or myocardial infarctions and no patients required target lesion revascularisation. One patient had mild angina which was being managed medically.
Repeat coronary angiography was performed in two patients. Patient 8 underwent angiography at six months because of a borderline positive ischaemia test in the setting of a known LAD lesion, although he was asymptomatic. The double stented region in the proximal RCA had mild in-stent restenosis with a late loss of 0.3 mm (9% stenosis) and the LAD lesion was judged to be angiographically insignificant. No further intervention was performed. Patient 13 underwent angiography five weeks following the original intervention because of an admission with troponin-negative atypical chest pain. There was no evidence of restenosis in the stented artery with a MLD identical to that at the end of the previous procedure.
Discussion
We describe a series of patients with suboptimal angiographic appearances following initial coronary stent deployment and high pressure balloon inflation that underwent deployment of a second stent within the first. In all cases the angiographic appearance was improved after placement of the second stent, with a decrease in mean stenosis from 34% to 10% and a 1 mm mean increase in MLD from 2.5 mm to 3.5 mm. Double stenting was not associated with procedural complications in any case and clinical follow-up with a mean duration of 19 months revealed no major adverse cardiac events.
Stent under expansion
Stent under expansion occurs due to either failure of the stent balloon to fully dilate the lesion or elastic recoil of the vessel wall causing partial collapse of the stent following deflation of the balloon. A small degree of stent recoil (10% reduction of MLD) is very common following stent placement5. More significant recoil has been related to lesions involving eccentric calcification. Vessel expansion occurs exclusively in the non-calcified part of the vessel wall, with the calcified region remaining unstretched6. Strut density is therefore also uneven with crowding of under expanded struts in the calcified segment and a sparse distribution of struts in the highly expanded non calcified segment. Following balloon deflation elastic recoil of the non-calcified vessel arc occurs due to low strut density and inadequate scaffolding7. The coronary ostia are particularly prone to stent recoil due to the fibroelastic properties of the aortic wall and the increased frequency of calcification8. This translates into a high restenosis rate despite large vessel diameter even with the use of DES9. Chronic stent recoil may contribute to this10.
Failure to fully dilate a lesion often coexists with acute stent recoil when fibrotic and/or calcific disease is stented. Calcific disease was detected angiographically in nine of the 10 patients labelled as acute stent recoil in this study. It is possible therefore that inadequate dilation contributed to the suboptimal stent expansion in these cases. However, as part of the patient selection in this study, we were careful to include only cases in which stent under expansion was attributed to stent recoil and in which full lesion dilatation by the stent balloon had been achieved as assessed angiographically. Lesion preparation frequently involved adjunctive devices and adequate lesion dilatation was ensured by high pressure post dilatation prior to placement of the second stent in all cases. This helps to explain the marked success of double stenting in this series as double stenting would not be expected to overcome a failure to dilate the lesion.
The mechanism via which double stenting improves angiographic outcomes is presumed to be at least partly due to an incremental increase in the degree of radial force exerted by the stent layer. All metallic stents exhibit a finite outwards radial force which can be quantified in comparative in vitro studies11,12. Although no in vitro studies of double stenting have been published, it is logical to assume that a double layer of stent struts will exhibit a greater degree of radial force than a single layer and would thus counteract vessel wall elastic recoil more effectively. As discussed above, in eccentrically calcified lesions stent recoil may occur due to an uneven distribution of stent struts. In these cases, deployment of a second stent will lead to more even strut coverage within the compliant segment which would be expected to result in improved scaffolding and reduced recoil.
Stent under expansion is a powerful predictor of adverse outcomes including increased risks of both restenosis and stent thrombosis2,13-16. This can be most accurately assessed using IVUS. A minimum stent area (MSA) <5 mm2 has been strongly associated with in-stent restenosis (ISR) for both de novo and restenotic lesions using DES17,18 as well as being highly associated with stent thrombosis19. MSA alone has been shown to be a more powerful predictor of stent failure than a value corrected for reference vessel area18. Although we did not use IVUS routinely in our study, inclusion required the finding of under expansion in two orthogonal views. An MSA of 5 mm2 corresponds to a mean diameter of 2.52 mm, which was not achieved following initial stent placement in 90% of patients with stent recoil in this study. Double stenting increased the MLD to greater than 2.52 mm in 8/9 of these cases with a mean increase in MLD of 0.9 mm (from 2.4 mm to 3.3 mm). The case with an initial MLD of greater than 2.52 mm involved incomplete expansion of an aorto-ostial lesion and was therefore also at high risk of restenosis: the MLD increased from 3.6 mm to 4.5 mm with double stenting. These improvements in MLD are likely to be important clinically. In the CRUISE study a 0.2 mm increase in mean MLD was associated with a significantly reduced risk of target vessel revascularisation at nine months4. Fujii and colleagues detected a MSA of <5 mm2 in 80% of patients with DES thrombosis compared with only 29% of controls in a case control study19. In a recently published analysis of patients from the ACUITY study, multivariate analysis showed that final stent MLD was an independent predictor of stent thrombosis with the difference in mean final MLD between the patients suffering stent thrombosis and controls being only 0.24 mm15.
Luminal filling defects
The second indication for double stenting in this study was to trap intra-stent filling defects due to either plaque prolapse or thrombus. Double stenting eliminated these filling defects in all three cases with an associated increase in MLD. Angiographically identified thrombus is known to be a risk factor for subsequent restenosis1 and stent thrombosis15. The clinical importance of plaque prolapse relates to the degree of prolapsed tissue. Although angiographically detectable plaque prolapse is rare, IVUS studies have shown that minor degrees of plaque prolapse are very common with both BMS20 and DES21,22 and do not appear to increase the risk of further events. In contrast, plaque prolapse leading to lumen compromise has been shown in an IVUS study to be a contributor to subacute stent thrombosis2, and in a postmortem study to be present in two fatal cases of late stent thrombosis23. Although high pressure non-compliant balloon inflation is usually performed for luminal filling defects, it may have little effect21. Similarly, thrombectomy devices may be used but are rarely successful when non-occlusive thrombus is present24.
Safety of overlapping stents
In terms of safety, we know from the management of ISR with further PCI that the presence of two completely overlapping stents within the coronary arteries is safe in the medium and long term, including with the use of DES25-28. Although the safety of double stenting within the same procedure has not been extensively studied, the effects of stent overlap have been investigated. Animal studies have detected poor endothelialisation of drug eluting stents, particularly at sites of stent overlap, raising concerns regarding the risk of stent thrombosis29-32. In the rabbit iliac model, delayed endothelialisation with DES was more evident at the site of overlap29,32, presumably as a result of the increased local concentration of drug. In contrast, in a pig coronary model whilst delayed endothelialisation was observed at the sites of DES overlap, complete coverage was achieved at 30 days30. Reassuringly from a clinical perspective, both randomised controlled trials33,34 and “real world” registry studies35 have shown that overlapping DES do not confer an increased risk of stent thrombosis or overall MACE in the short to medium term. Pooled IVUS analysis from the TAXUS trials showed significant reductions in restenosis in the overlap regions as compared with overlapping BMS36. There was no increased risk of expansive remodelling in these overlapping stent strut regions and there were no cases of acquired incomplete stent apposition in the overlap regions37. A pooled analysis of studies involving overlapping Cypher sirolimus-eluting stents (Cordis, Johnson & Johnson, Warren, NJ, USA) showed this practice to be safe with no increase in MACE as compared with BMS38. Therefore, it appears that the concerns regarding overlapping stents derived from animal studies do not translate into detectable adverse outcomes in clinical studies, albeit with a shorter stent overlap (mean 6.3 mm in the TAXUS trials) compared to our study (mean 11 mm).
Previous studies of double stenting
Several small studies have reported the use of double stenting. Morton and Gunn reported on 18 patients treated with BMS double stenting39. The majority of these patients were treated for plaque prolapse and three patients had ostial lesions. Three patients (17%) required target vessel revascularisation (TVR) within a mean follow-up of 21 months, although only one of these patients developed restenosis within the double stented segment, which was ostial in nature. There were no deaths or myocardial infarctions (MIs) at six months follow-up. Morici and colleagues reported 10 cases of DES double stenting, performed in nine cases to reduce an assumed high risk of subsequent restenosis rather than because of a suboptimal result following the first stent deployment40. There were no deaths, MIs or stent thrombosis at a mean follow-up of 10.1 months, albeit with a target lesion revascularisation (TLR) rate due to restenosis of 30%. In addition there are several case reports and small series reporting double stenting, predominantly using BMS for the treatment of ostial disease and plaque prolapse with variable follow-up6,41-47. These reports have not described any adverse events following double stenting.
An alternative to double stenting with two DES may be to deploy a DES and a BMS aiming to simultaneously reduce restenosis and avoid late complications related to drug toxicity. However, a recent study of overlapping BMS and DES for long lesions showed a high incidence of diffuse ISR within the BMS treated segments, including the overlap region48, and it is unclear whether this practice represents a better option than overlapping DES.
Limitations
The lack of a control group is the most important limitation of our study. However a suboptimal angiographic appearance post-stenting is a rare occurrence, as evidenced by the small number of patients in our series, and it is extremely difficult to identify accurately matched control cases who were left with a residual poor angiographic appearance at the end of their procedure. A further drawback is that angiographic follow-up was not obtained in all patients. However this series represents a “real-world” sample of patients and routine surveillance angiography is not performed in our centre. Finally, although it is reassuring that there were no MACE during the medium-term follow-up, the small number of patients in this series precludes any definitive statement on the long-term safety of double-stenting.
Conclusion
Acute recoil and luminal filling defects following stent deployment are associated with an increased risk of adverse events. We have demonstrated in this small series that the procedure of double stenting with drug eluting stents can significantly improve angiographic outcome in these cases with excellent clinical results in the medium-term.