
Abstract
Aims: In the last 10 years, cryotherapy has been investigated as a new technology to treat vascular disease. The efficiency of cryotherapy in stabilising atherosclerotic plaques has never been described. The purpose of the present study was to evaluate the effect of catheter-based cryotherapy on atherosclerotic plaque composition in a rabbit model of atherosclerosis.
Methods and results: Twenty-four New Zealand white rabbits were fed a 0.3% cholesterol-supplemented diet for 24 weeks. At two predefined sites of the atherosclerotic thoracic aorta, catheter-based cryotherapy, applying either single-dose, double-dose cryotherapy or control inflation, was performed after randomisation. Rabbits were continued on a cholesterol-supplemented diet for one day (acute) or four weeks (chronic). One day after cryotherapy, apoptotic cell death of smooth muscle cells (SMCs) and endothelial cells (ECs) was observed, whereas macrophages were unaffected. Four weeks later, the amount of SMCs was restored, the EC layer was regenerated, and a subendothelial macrophage-free layer was formed, indicative of a more stable plaque. In addition, both the thickness and the type I collagen content of the fibrous cap were increased.
Conclusions: The present study demonstrated that cryotherapy is feasible and appears to stabilise atherosclerotic plaques in a rabbit model.
Introduction
Atherosclerosis is a progressive inflammatory disease of the large and medium-sized arteries and is characterised by the formation of plaques in the vessel wall. It is the main cause of death in the Western world and its incidence is still increasing1. During the development of the disease not only the size of the plaque but, more importantly, also the stability play a major role. Unstable plaques are characterised by a large necrotic core, a high infiltration of inflammatory macrophages and a thin fibrous cap with few smooth muscle cells (SMCs), and a low collagen content. When a plaque develops an unstable phenotype it may easily rupture, followed by thrombosis, myocardial infarction, stroke or sudden death1-3. Despite the therapeutic advances in cardiovascular research, plaque rupture remains the leading cause of acute cardiovascular syndromes. Therefore, the need for the development of plaque-stabilising interventions is high.
Over the last decade, there has been interest in attempting to use cryoenergy as a cryotherapeutic alternative to treat vascular tissue. The use of cryoenergy in the cardiovascular system began in 1977 when it was used to treat cardiac arrhythmias surgically4. Over subsequent years, it became widely recognised that cryoenergy was the energy source of choice in cardiac ablations. Its safety and efficacy were unsurpassed as surgeons were able to use it to ablate delicate cardiac structures such as the atrioventricular (AV) node without concern for thrombosis, perforation or other adverse events5. With the advent of radiofrequency catheter technology in the 1980s and cryo catheters in 1998 it became clear that cryoenergy was the safest energy source when working inside a beating heart6. Prior studies have also indicated that cryotherapy can have beneficial effects on vascular plaque by increasing its collagen content. Tanguay et al showed that intravascular cryotherapy in normal pig carotid arteries caused an increase in collagen and positive remodelling without compromising the patency of the vessel since no late luminal loss was observed7. Similarly, increased wall thickness and collagen content in cryotherapy-treated arteries has been reported8. However, the safety and efficacy of cryotherapy on stabilising atherosclerotic plaques have never been described. Therefore, the purpose of the present study was to evaluate the biological response to cryotherapy using the Generation II CryoTherapeutics system (CryoTherapeutics GmbH, Cologne, Germany) in a rabbit model of atherosclerosis. We examined the effects of a single and double cryo dose on plaque composition in the aorta at an acute (18-24 hrs) and at a chronic (28 days) time point.
Methods
IN VIVO EXPERIMENTS AND CRYOTHERAPY
Twenty-four New Zealand white rabbits (obtained from the University of Gent, Merelbeke, Belgium) were fed a 0.3% cholesterol-supplemented diet for 24 weeks to induce non-flow-limiting atherosclerotic plaques in the aorta. All experiments conformed to the guiding principles of the Declaration of Helsinki and were approved by the Ethical Committee of the University of Antwerp. At the index procedure, the marginal ear vein was cannulated and the rabbit was anaesthetised with sodium pentobarbital (30 mg/kg, intravenously). The right carotid artery was dissected under sterile conditions and a 6 Fr sheath was introduced. Under fluoroscopy, a 0.014” guidewire (WHISPER; Abbott Vascular, Santa Clara, CA, USA) was positioned in the right or left iliac artery and a single dose of aspirin (60 mg/kg) as well as heparin (100 IU/kg) was administered. At two predefined sites within the descending thoracic aorta (with rib number used as identifying mark), and at a distance of a minimum of 1 cm from each other (to avoid overlap), catheter-based cryotherapy was applied. According to a randomisation scheme, either single-dose, double-dose (i.e., two subsequent inflations) cryotherapy or control inflation was performed (Online Figure 1). After recovery, the rabbits were continued on a 0.3% cholesterol-supplemented diet for one day (acute) or four weeks (chronic) to evaluate the response to cryotherapy.
CRYOTHERAPY SYSTEM
The cryotherapy system (Figure 1A) used in this animal study was composed of a chiller unit which cooled a refrigerant fluid to –70°C using a slurry of dry ice pellets and 99.5% isopropanol. The refrigerant fluid cooled the distal end of a balloon catheter to a temperature of –10°C to –15°C. A pressure source was used to pump the refrigerant fluid to the distal end of the balloon catheter.
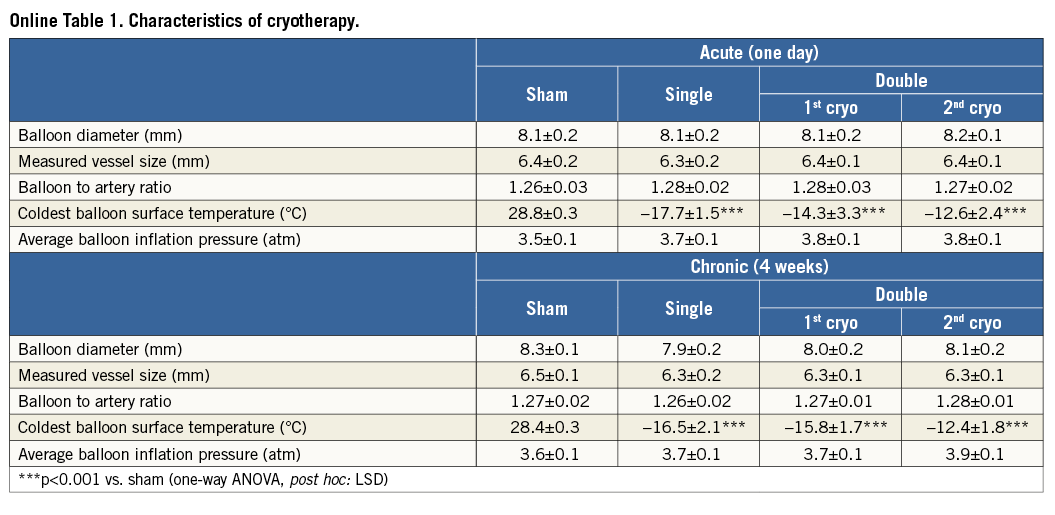
The cryo catheter (Figure 1B, Figure 1C, Figure 1D) was constructed with a thermocouple to measure the refrigerant fluid temperature as it was injected into the catheter, and three additional thermocouples on the surface of the balloon of the catheter which measured the temperature of the surface at three different points. All ethylene oxide-sterilised balloon catheters had a balloon length of 15 mm. A diameter of 8 mm was chosen to achieve a balloon to artery ratio of approximately 1.2-1.3, as measured by quantitative angiography (Online Table 1). The slightly oversized balloon was inflated at low pressures (±3.7 atm) (Online Table 1), to ensure proper wall apposition of the catheter. The cryo catheter was attached to the chiller unit on the table and was connected to the console via two umbilicals, both 75 cm in length. One umbilical was used for refrigerant fluid return and the other for electrical and pressure sensing.
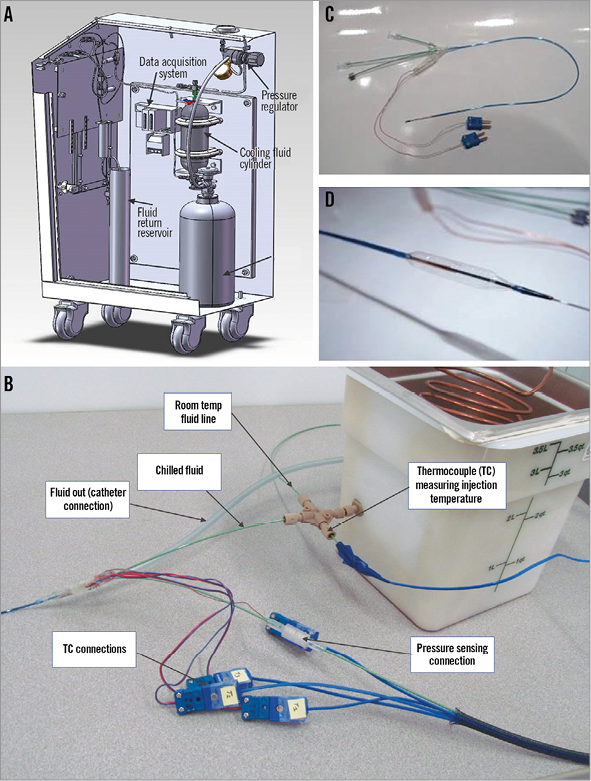
Figure 1. The cryotherapy system and balloon catheter. The console delivers high-pressure fluid to the chiller unit (A). Nitrogen gas from the N2 cylinder pressurises the cooling fluid in the cylinder. The pressurised fluid exits the console via the injection umbilical and enters the chiller unit. The fluid is chilled to –70°C and enters the catheter (B & C). Cold fluid is injected inside the balloon and cools the outer surface (D). Fluid returns back through the catheter shaft inside the console and drains into a reservoir container. A pressure regulator adjusts nitrogen gas pressure and fluid flow. A second injection line from the console is connected to the catheter, bypassing the chiller unit, allowing injection of warm (20°C) fluid inside the balloon (control applications). Three thermocouples (TC) measure the temperature of the outside surface of the balloon and one thermocouple monitors the fluid temperature inside the balloon (B). Four wires extend back to the connectors and electrical umbilical to the console data acquisition system (A). A static tube inside the catheter transmits the balloon pressure to a transducer inside the console via a pressure-sensing umbilical. The pressure transducer is connected to the data acquisition system, which also records the fluid injection temperature by a thermocouple placed after the chiller unit. A pressure transducer in the console transmits injection pressure data to the data acquisition system.
HISTOLOGY
After euthanasia, aortas were flushed with saline. The rib number served as an identifying mark to harvest the aortic segments of interest correctly (treated with single- or double-dose cryotherapy, sham balloon, or naive control, i.e., a proximal adjacent untreated atherosclerotic segment). They were placed in 4% formalin for 24 hrs or frozen at –80°C. Vessels were paraffin- or optimal cutting temperature (OCT)-embedded and stained with haematoxylin-eosin or Sirius Red (collagen stain) for histopathological analysis. Immunohistochemical detection of macrophages (Monoclonal Mouse Anti-Human CD68, Clone PG-M1; Dako, Agilent Technologies, Glostrup, Denmark), SMCs (anti-α-SMC actin, clone 1A4; Sigma-Aldrich, St. Louis, MO, USA), endothelial cells (Von Willebrand factor, sheep polyclonal anti-VWF; Binding Site, San Diego, CA, USA), vascular adhesion molecule-1 (VCAM-1, rat anti-CD106 antibody; Bd Biosciences, San Jose, CA, USA), and caspase activation (FITC-labelled z-Val-Ala-dl-Asp[O-methyl]-fluoromethylketone [zVAD]) was carried out by the indirect peroxidase antibody conjugate technique9-11. Vectastain® (Vector Laboratories, Burlingame, CA, USA), antibodies conjugated with horseradish peroxidase (Dako) or anti-FITC (Dako) were used as secondary antibodies. For detection of oligonucleosomal DNA cleavage, a stringent Terminal deoxynucleotidyl transferase dUTP Nick-End Labelling (TUNEL) technique was used9. Morphometric analysis was performed by two experienced and blinded operators using an image analysis system (ImageJ, NIH), as previously described10. The % alpha-SMC actin in the plaque, the % collagen type I in the plaque, and the % collagen type I in the fibrous cap were measured in a complete cross-section of the plaque or fibrous cap. The % macrophages in deep layers of the plaque were measured in the abluminal half of the plaque. The length of VWF positive cells along the luminal border was quantified in a complete cross-section of the thoracic aorta and expressed as a percentage of the total perimeter. VCAM-1 positivity was expressed as percentage of VWF positivity. All these morphometric parameters were normalised to the untreated atherosclerotic segment (adjacent to the sham or cryo-treated segment) (Online Figure 1) to take into account the variability in rabbit plaque formation. The thickness of the fibrous cap was analysed on anti-α smooth muscle actin stained sections (10 measurements per plaque), and was normalised to plaque thickness. The thickness of the subendothelial macrophage-free layer was measured perpendicular from the endothelial cells up to the region where macrophages appeared in the plaque, and was normalised to plaque thickness. Because untreated atherosclerotic segments were caspase negative, normalisation could not be performed for this parameter.
STATISTICAL ANALYSIS
Values were expressed as mean±SEM. Statistical analyses were performed using SPSS software, Version 22 (IBM Corp., Armonk, NY, USA). Analysis of variance (ANOVA), followed by the LSD (least significant difference) post hoc test, was used to compare treatment groups. For the analysis of the zVAD stain, the Mann-Whitney test was applied. The number of calcified plaques was compared by Pearson’s chi-square test. Differences were considered significant at p<0.05.
Results
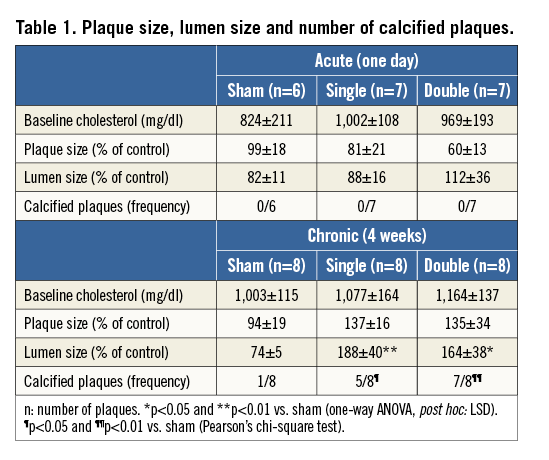
Rabbits (3-3.5 kg) had cholesterol levels of 1,016±88 mg/dl at the index procedure, i.e., after cholesterol feeding for 24 weeks. The baseline plasma cholesterol was equal in the three treatment groups (sham, single and double cryotherapy) in both acute and chronic conditions (Table 1). All animals survived the cryotherapy procedure, confirming the feasibility of cryotherapy. Two rabbits from the acute treatment group were excluded because plaque development was lacking (non-responders with cholesterol levels below 100 mg/dl).
APOPTOSIS
One day after treatment, both the single (3.17±1.05%, Mann-Whitney; p=0.044 vs. sham) and double (3.15±1.48%, Mann-Whitney; p=0.032 vs. sham) cryo dose increased the caspase positive area in the plaque as compared to sham (0.32±0.17%), indicating apoptosis induction (Figure 2A). In addition, TUNEL staining was present in plaques of single- and double-dose sections but not in sham-treated plaques, showing clear signs of nuclear fragmentation (Figure 2B). Four weeks after cryotherapy, apoptosis was absent, as indicated by negative zVAD (Figure 2A) and TUNEL stains (data not shown).
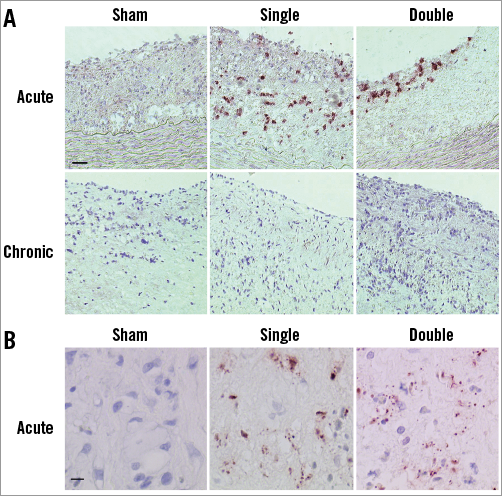
Figure 2. Presence of apoptosis. A) One day after single and double cryotherapy apoptotic cells were present. In chronic conditions zVAD staining was negative, indicating that caspase activity was absent. Scale bar=100 µm. B) TUNEL staining showed nuclear fragmentation after single and double cryotherapy in acute conditions. Scale bar=10 µm.
VWF AND VCAM-1 EXPRESSION IN ENDOTHELIAL CELLS (ECs)
One day after the single or double cryo treatment, the amount of VWF-positive ECs was decreased compared to sham-treated plaques (Figure 3A, Figure 3B). The remaining ECs showed a trend towards increased VCAM-1 expression in single (14±9%) and double (20±9%) cryo-treated sections as compared to sham (8±4%, one-way ANOVA; not significant). After four weeks, the EC layer was restored (no difference with sham) (Figure 3A, Figure 3B). Moreover, VCAM-1 expression of the ECs was normalised to baseline levels (single cryo dose: 5±2%; double cryo dose: 2±1%; sham: 3±2%, one-way ANOVA; not significant).
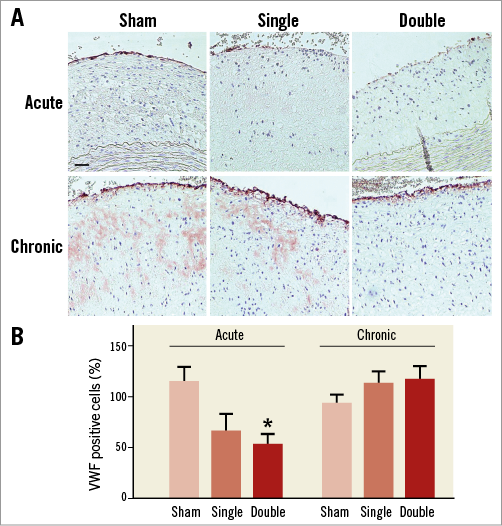
Figure 3. VWF staining of atherosclerotic plaques. A) Photomicrographs of VWF-positive ECs. B) Quantification of histological data showed that cryotherapy resulted in a decrease in ECs, which was more pronounced after double cryo treatment (n=6-7, one-way ANOVA, post hoc LSD: *p<0.05 vs. sham). However, four weeks later the ECs were regenerated (n=8, one-way ANOVA: not significant). Scale bar=100 µm.
SMOOTH MUSCLE CELLS (SMCs)
One day after the procedure, both single- and double-dose cryotherapy resulted in a significant decrease in the amount of SMCs in the atherosclerotic plaques. Importantly, 28 days after the procedure, the SMC content in both cryo groups was fully restored (Figure 4A, Figure 4B). Four weeks after single or double cryotherapy, the thickness of the fibrous cap was increased (Figure 4C).
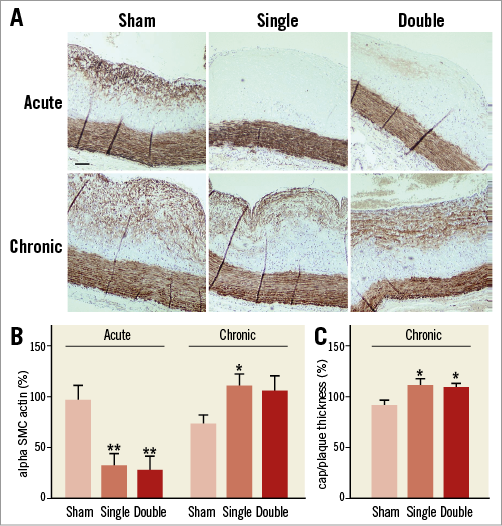
Figure 4. Alpha smooth muscle cell actin staining of atherosclerotic plaques. A) Cryotherapy resulted in an acute loss of SMCs in the plaque. Four weeks later, the amount of SMCs was restored. Scale bar=100 µm. B) Quantification of histological images confirmed the significant decrease in SMC content one day after single or double cryotherapy (n=6-7, one-way ANOVA, post hoc LSD: **p<0.01 vs. sham). One month after cryotherapy, the amount of SMCs was restored (n=8, one-way ANOVA, post hoc LSD: *p<0.05 vs. sham). C) The ratio of fibrous cap thickness/plaque thickness was increased four weeks after single or double cryotherapy (n=8, one-way ANOVA, post hoc LSD: *p<0.05 vs. sham).
TYPE I COLLAGEN
In acute and chronic conditions, the total type I collagen content in the plaque was unaltered both in single and double cryotherapy sections as compared to sham (Figure 5A, Figure 5B). However, in the fibrous cap, the amount of type I collagen was significantly increased by double cryo treatment (Figure 5C).
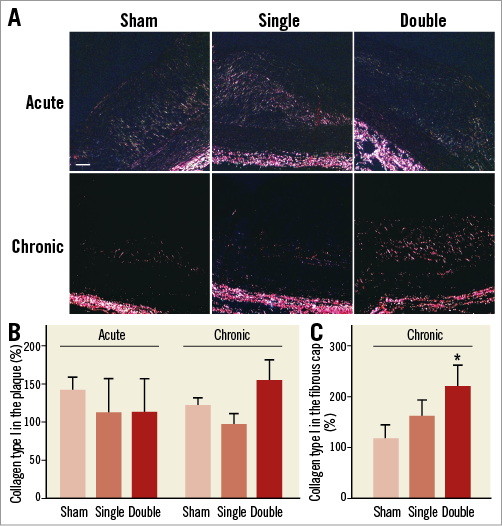
Figure 5. Sirius Red staining of atherosclerotic plaques. A) Photomicrographs of type I collagen. B) Quantification of histological images showed no difference at acute conditions (n=6-7, one-way ANOVA: not significant). Double cryotherapy tended to increase type I collagen in the plaque at the chronic time point (n=8, one-way ANOVA: not significant). Scale bar=100 µm. C) In the fibrous cap, type I collagen was significantly increased four weeks after double cryotherapy (n=8; one-way ANOVA, post hoc LSD: *p<0.05).
MACROPHAGES
At the acute time point, the amount of macrophages in the plaque was not different among both cryo treatments and sham (Figure 6A). Importantly, four weeks after single or double cryotherapy, a subendothelial macrophage-free layer was formed (Figure 6B). In the deep layers of the plaque, adjacent to the media, the amount of macrophages was significantly increased after single cryotherapy (Figure 6C).
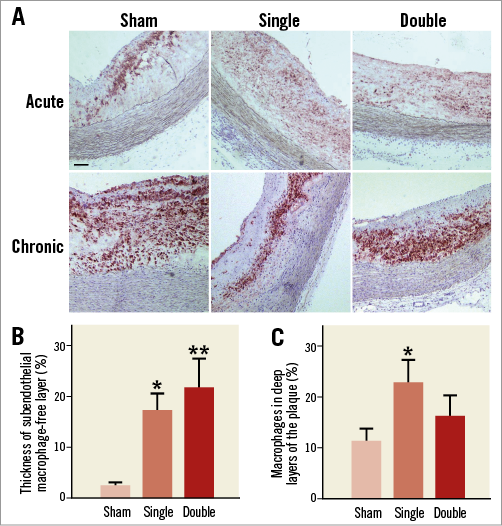
Figure 6. CD68 staining of atherosclerotic plaques. A) Cryotherapy did not affect the total amount of macrophages present in the atherosclerotic plaques in acute conditions (n=6-7, one-way ANOVA: not significant). However, four weeks after cryotherapy it appeared that macrophages had migrated from luminal to deep layers of the plaque (n=7, one-way ANOVA: not significant). Scale bar=100 µm. B) A subendothelial macrophage-free layer was formed four weeks after cryotherapy (n=7; one-way ANOVA, post hoc LSD: *p<0.05; **p<0.01 vs. sham). C) The amount of macrophages in deep layers of the plaque was increased after single cryotherapy (n=7; one-way ANOVA, post hoc LSD: *p<0.05 vs. sham).
PLAQUE AND LUMEN SIZE
One day after single or double cryotherapy, plaque and lumen size were not different from sham-treated sections (Table 1, Online Figure 2). Four weeks later, there was a slight but not significant increase in plaque size. Aortic lumen was significantly increased in both single and double cryo dose groups versus sham (Table 1, Online Figure 2), indicating positive remodelling.
PLAQUE CALCIFICATION
Calcifications were not detected one day after cryotherapy. Four weeks later, macro-calcifications could be clearly observed in single and double cryo dose-treated plaques near the media. The frequency of plaque calcification was significantly elevated in both cryo groups as compared to sham (Figure 7, Table 1).
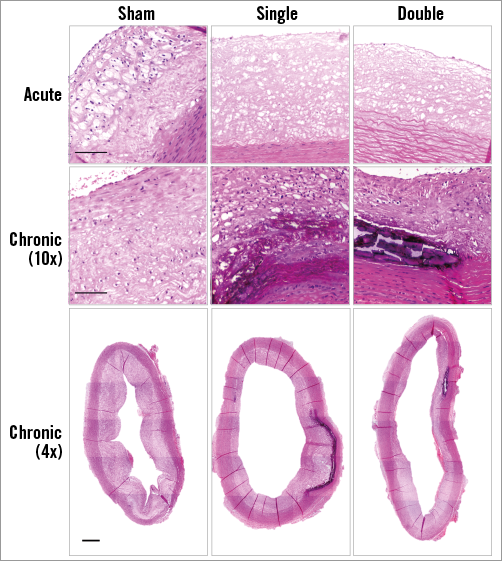
Figure 7. Plaque calcifications. One day after cryotherapy, no calcifications were observed in haematoxylin/eosin stained sections. Four weeks later, the presence of calcified plaques was markedly increased as compared to sham treatment. Scale bar=100 µm (high magnification) and 500 µm (low magnification).
Discussion
In the last 10 years, cryotherapy has been investigated as a new technology to treat and prevent vascular and arrhythmic disease. Previous studies focused mainly on the treatment and prevention of stenosis and restenosis in peripheral arterial disease and determined the effect of cryotherapy on vascular remodelling and collagen content of balloon-injured arteries7,8,11. The efficacy of cryotherapy to stabilise atherosclerotic plaques has never been described. Therefore, we investigated the effects of cryotherapy on plaque composition in the aorta of cholesterol-fed rabbits. One day after cryotherapy, SMCs and ECs underwent apoptosis, whereas macrophages were unaffected. One month later, a subendothelial macrophage-free layer was formed which is, together with the increased collagen I content of the fibrous cap, indicative of a more stable plaque phenotype.
APOPTOSIS AND REPAIR OF ENDOTHELIAL AND SMOOTH MUSCLE CELLS
We report apoptotic cell death of ECs and SMCs one day after single or double cryotherapy, confirming previous findings in non-atherosclerotic arteries12-14. ECs appear to be more resistant to cryotherapy as compared to SMCs12,13,15, which was also seen in our study. At the acute time point, the amount of SMCs present in the plaque was more than 70% decreased. A decline was also observed in ECs, but this was never higher than 50%. The apoptotic cell death of smooth muscle cells and endothelial cells one day after cryotherapy might lead to plaque instability, plaque rupture and thrombotic complications in patients. Therefore, the safety of the acute effects of this procedure needs to be evaluated in future trials. To prevent a possible thrombotic occlusion, dual antiplatelet therapy or the use of an anticoagulant might be a solution. However, in the present rabbit study we did not observe death as a consequence of acute cryotherapy.
Four weeks after cryotherapy, apoptosis was no longer observed. Moreover, the EC layer was regenerated and the amount of SMCs was restored. These findings indicate that the initial apoptotic cell death of ECs and SMCs, which might promote plaque instability, is repaired within 28 days after cryotherapy.
The ECs at the acute time point showed a trend towards higher expression levels of VCAM-1, indicating that these cells might be more activated and have adopted a more inflammatory phenotype. A clinical trial of Wildgruber et al16, in which the effects of cryotherapy on the femoral artery were explored, showed increased levels of ICAM-1, VCAM-1, E-selectin and monocyte chemoattractant protein-1 (MCP-1). In that study, VCAM-1 expression started to increase 1 hr after cryotherapy and reached the highest levels four weeks later. However, in the present study we found that VCAM-1 expression was brought back to baseline conditions one month after cryotherapy. Thus, four weeks after cryotherapy ECs delineating an atherosclerotic plaque have a normalised anti-inflammatory phenotype and will not contribute to plaque destabilisation.
FIBROUS CAP THICKNESS AND TYPE I COLLAGEN CONTENT
A thick fibrous cap and the presence of type I collagen is essential for plaque stability. We observed an increase in cap thickness of 20% in both single and double cryo-treated sections four weeks after cryo treatment, which is a novel finding. Despite the fact that cryotherapy is known to increase the synthesis of type I and type III collagen in the vessel wall7,8,11, this was never demonstrated in atherosclerotic plaques. In the present study, the amount of type I collagen in the fibrous cap was increased, in particular by the double cryo dose, indicating that plaques can evolve to a more stable phenotype by cryotherapy.
INFLAMMATION
One day after cryotherapy, the total amount of macrophages in atherosclerotic plaques was not altered. However, one month later a subendothelial macrophage-free layer was formed, which is favourable for plaque stability. A lower amount of macrophages in the luminal region of atherosclerotic plaques will result in less damage to the fibrous cap. This was reflected by an increase not only in the thickness of the fibrous cap but also in its amount of type I collagen.
In contrast, the amount of macrophages in the deep layers of plaques was increased, suggesting that after cryotherapy macrophages migrated from luminal to deep plaque layers. The macrophages were probably attracted by apoptotic SMCs in the deep layers of the plaque8. Nevertheless, the contribution to plaque instability of an increased macrophage content in deep plaque layers seems to be minimal, because both fibrous cap thickness and the amount of type I collagen in the fibrous cap were increased after cryotherapy.
POSITIVE REMODELLING
The present study revealed signs of positive remodelling four weeks after cryotherapy. A study by Cheema et al, showed a larger lumen area in rabbit iliac arteries 72 hrs after cryotherapy, but these beneficial effects were not observed after 10 weeks due to a fibrotic response8. This different finding can be explained by the fact that cryotherapy was applied directly on the vessel wall, since no atherosclerotic plaques were present8. Furthermore, the lower cryo temperatures (–30°C to –60°C) and higher balloon pressures used in the study by Cheema et al resulted in extensive damage of the vessel wall8. In the present study, a milder cryo protocol was applied on atherosclerotic plaques in the thoracic aorta, leading to a larger lumen area four weeks after cryotherapy. Our data are confirmed by previous research reporting positive remodelling of swine carotid and femoral arteries7,11. Thus, the outward remodelling after cryotherapy might prevent restenosis frequently associated with balloon angioplasty.
Several studies describe outward remodelling as a characteristic of unstable plaques17,18, but we presume that it is important to differentiate between spontaneous and induced positive remodelling. We observed induced positive remodelling in combination with more stable plaques, because type I collagen in the plaques and fibrous cap thickness were increased, indicating that induction of outward remodelling by cryotherapy does not lead to plaque destabilisation after four weeks.
CALCIFICATION
Cryotherapy resulted in an increased number of macro-calcifications present in the plaques, which was more pronounced in the double-dose group as compared to the single-dose group. Cheema et al8 showed cartilage, bone formation and calcification in the media of cryotherapy-treated iliac arteries. Previous work showed that remnants of apoptotic SMCs remain in the plaque as matrix vesicles and may act as nucleating structures for calcification19,20. Since the present study observed apoptotic cell death of SMCs, this could be the initiating process for bone formation. Moreover, bone morphogenetic protein (BMP)-2, which is able to enhance bone formation, has been described as being present in calcified regions of the vessel wall after cryotherapy8. BMP-2 is expressed in both SMCs and macrophages21,22. Interestingly, macrophages are able to enhance the expression of BMP-2 in SMCs and therefore play an important role in plaque calcification21. In the present study, we observed that the calcified regions were situated in the deep layers of the plaque and that these regions were associated with an increased amount of macrophages. Therefore, we hypothesise that the macrophages were attracted by apoptotic cell death of SMCs. The matrix vesicles of these SMCs initiated calcification and the attracted macrophages accelerated the process.
Although it is known that coronary artery calcification is a predictor of cardiovascular events23,24, the direct link between Ca++ and plaque rupture is still controversial and not fully elucidated. Previous research has shown that calcification does not decrease the mechanical stability of coronary plaques25. The presence of macro-calcifications is probably the result of a healing process that is able to separate the inflammatory plaque content from the vessel lumen26,27. Moreover, plaque calcification has been associated with decreased fibrous cap inflammation and enhanced plaque stability28. Also, the calcification pattern plays an important role, because “spotty” calcifications are associated with a higher incidence of cardiovascular events. In contrast, we observed a dense calcification pattern, indicative of lower-risk plaques27,29,30. In general, stable plaques show greater calcifications than unstable plaques31. Therefore, we believe that the presence of plaque macro-calcifications after cryotherapy may have a plaque stabilising effect.
STUDY LIMITATIONS
In the present study, the effects of cryotherapy were investigated in the thoracic aorta of atherosclerotic rabbits. Besides the limitations of this model, a rather small number of rabbits was included. The effect of cryotherapy on different vascular beds using a large animal model, such as the pig, may need to be examined. Moreover, we investigated the chronic effects of cryotherapy after a period of four weeks, which might have been too short to study long-term effects on atherosclerotic plaque stability. Finally, only morphometry was used to measure lumen size, which has drawbacks associated with tissue processing, such as vessel shrinkage. This issue could have been avoided by using intravascular imaging at baseline and after cryotherapy.
Conclusion
Cryotherapy led to the formation of a subendothelial macrophage-free layer and a thicker fibrous cap with more type I collagen as compared to sham, indicative of a more stable plaque. These beneficial results were more pronounced after double cryo treatment. The higher frequency of calcified plaques due to cryotherapy needs further investigation, especially concerning the influence on plaque stability. The present study demonstrated that cryotherapy is feasible and appears to be a promising technique to stabilise atherosclerotic plaques.
| Impact on daily practice We investigated whether cryotherapy affects plaque composition in the thoracic aorta of cholesterol-fed rabbits. One day after cryotherapy, ECs and plaque SMCs underwent apoptosis, whereas macrophages were unaffected. One month later, a subendothelial macrophage-free layer was formed, which together with increased collagen I content of the fibrous cap is favourable for plaque stability. While these observations have been made in a rabbit model, taking into account the limitations of such a model, we believe that this application could have a potential benefit when these findings are translated into a clinical study. |
Acknowledgements
The authors thank Lina Maria Zuleta Zamorano and Slim Louizi, engineers from CryoTherapeutics, for their help with the cryo procedures. We also acknowledge Rita Van Den Bossche and Anne-Elise Van Hoydonck for technical support.
Funding
This study was funded by CryoTherapeutics GmbH, Cologne, Germany.
Conflict of interest statement
D. Nahon, M. Buchbinder and J. Yianni are shareholders of CryoTherapeutics. D. Santoianni is an employee of CryoTherapeutics. The other authors have no conflicts of interest to declare.
Supplementary data
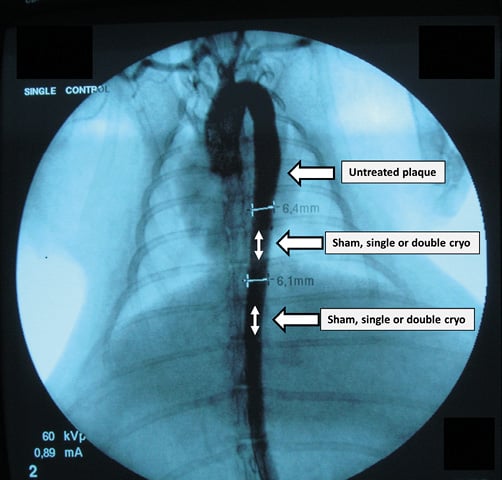
Online Figure 1. Aortogram. Under fluoroscopy, a 0.014” guidewire was positioned in the right or left iliac artery. At two predefined sites (white double-headed arrows) within the descending thoracic aorta (with rib number used as identifying mark) and at a distance of minimum 1 cm from each other (to avoid overlap), catheter-based cryotherapy was applied. According to a randomisation scheme, either single-dose, double-dose (i.e., two subsequent inflations) cryotherapy or control inflation was performed. One day or four weeks later, the rib number served as an identifying mark to harvest the aortic segments of interest correctly (treated with single- or double-dose cryotherapy or sham balloon). Also, a naive control, i.e., a proximal adjacent untreated atherosclerotic segment, was collected (arrow, untreated plaque).
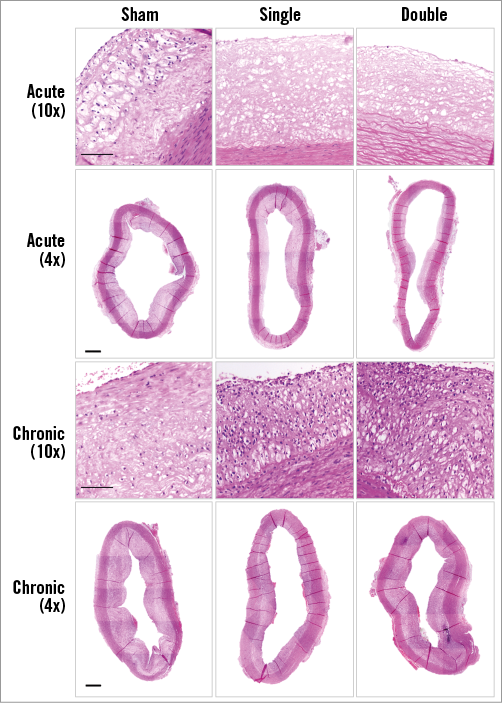
Online Figure 2. Atherosclerotic plaques. High- and low-power micrographs (H/E) to illustrate the overall aortic atherosclerotic lesion. Scale bar=100 µm (high magnification) and 500 µm (low magnification).

