
Abstract
Aims: Coronary intravascular lithotripsy (IVL) is a novel approach to vascular calcium modification that restores vessel compliance allowing effective lesion expansion. In this study we report the capacity for coronary IVL to precipitate ventricular ectopics (“shocktopics”) and asynchronous cardiac pacing.
Methods and results: This was a retrospective review of all cases of coronary IVL (n=54) undertaken in the Royal Victoria Hospital, Belfast, between September 2018 and March 2019. The indication for PCI was chronic stable angina in 46.1% (n=26), non-ST-elevation acute coronary syndrome (NSTEACS) in 33.3% (n=18) and ST-elevation myocardial infarction (STEMI) in 18.5% (n=10) of patients. The incidence of coronary IVL-provoked ventricular capture was 77.8% (n=42). Multivariable logistic regression analysis identified heart rate as the only independent predictor of an increased risk of IVL-induced ventricular capture. Patients with a heart rate <65 bpm prior to IVL were sixteen-fold more likely (OR 16.3 [2.4-110.8], p=0.004) to experience events compared to patients with a heart rate ≥65 bpm. “Shocktopic” beat morphology was largely uniform in each patient and appeared dependent on the target lesion location, in keeping with mechano-electric coupling through activation of local stretch-activated cardiomyocyte channels. No adverse clinical events occurred as a result of coronary IVL-induced capture.
Conclusions: Coronary IVL with the Shockwave Medical system is associated with a high incidence of “shocktopics” and asynchronous cardiac pacing that is largely dependent on the resting heart rate. There have been no clinical events associated with this phenomenon, but further systematic evaluation is warranted.
Introduction
Percutaneous coronary intervention (PCI) is one of the most commonly performed medical procedures worldwide1. Coronary artery calcification is frequently encountered and remains a major predictor of PCI failure2,3,4. Traditionally, high-pressure balloons, scoring and cutting balloons, and rotational atherectomy have been used to overcome calcific disease3. However, these treatments are of limited success because of their inability to differentiate between calcific and soft tissue or failure to modify deeper-lying calcium5,6.
To address these limitations, the coronary intravascular lithotripsy (IVL) system (Shockwave Medical, Santa Clara, CA, USA) was developed. Emitters located within an angioplasty balloon deliver pulsatile mechanical energy in the form of acoustic pressure waves (shock waves) for selective disruption of calcium within the vessel wall. The aim of resulting microfractures is to restore vessel compliance and permit effective lesion preparation to be performed safely at low pressure7.
In this report, we highlight the capacity of coronary IVL to precipitate ventricular ectopics (“shocktopics”) and/or asynchronous cardiac pacing during treatment. The incidence and predictors of ventricular capture are examined together with the potential implications of these findings, including the theoretical risk of provoking sustained ventricular dysrhythmia and the possible impact on implanted pacemaker function.
Methods
STUDY DESIGN AND PATIENT POPULATION
This was a retrospective review of all cases of coronary IVL undertaken in the Royal Victoria Hospital, Belfast, between September 2018 and March 2019. No patients were excluded. All patients had a clinical indication for revascularisation and underwent coronary IVL because of non-dilatable coronary artery disease with concentric calcification identified on angiography and/or intravascular imaging. All patients received coronary IVL as part of standard care and provided written informed consent prior to undergoing PCI.
CORONARY IVL
All patients received coronary IVL as per manufacturer recommendations. The Shockwave Medical coronary IVL system integrates multiple emitters within an angioplasty balloon. The balloon is positioned over a standard coronary wire within the target lesion and inflated to 4 atm to ensure vessel wall apposition. Pulsatile sonic pressure waves are then delivered locally at a rate of one pulse per second for up to 10 seconds. The process is repeated (up to a maximum of 80 pulses) until the lesion is adequately prepared for stent deployment. At 3 mm from source, the energy density is 9.6 (±1.6) x 10−3 mJ/mm2.
EVENTS AND DEFINITIONS
Eight-lead continuous electrocardiogram (ECG) recordings from each patient were reviewed for evidence of coronary IVL-induced “shocktopics” and/or asynchronous cardiac pacing by two cardiologists. Ventricular capture was identified as a change in QRS morphology with the onset precisely coinciding with the electromagnetic (EM) “spike” of the shockwave pulse. A “shocktopic” was defined as an isolated ventricular capture beat. Asynchronous cardiac pacing was defined as ≥2 consecutive ventricular capture beats. In the event of disagreement, capture was assumed not to have occurred. ECG recordings were also scrutinised for evidence of “shocktopics” triggering atrial or ventricular tachyarrhythmia including non-sustained and sustained ventricular tachycardia (VT) or ventricular fibrillation (VF).
STATISTICAL ANALYSES
Continuous variables are expressed as mean±standard deviation (SD) and were compared using the unpaired t-test. Categorical variables are expressed as percentages and were compared by the chi-square test or Fisher’s exact test as appropriate. Independent predictors of ventricular capture were determined by multivariable logistic regression. Variables included in the final model were selected by stepwise regression using Akaike information criteria. Candidate variables (age, previous myocardial infarction [MI], primary PCI, IVL balloon location, resting heart rate ≤65, creatinine, QTc) were selected on the basis of differences between the groups. Two-sided p-values of ≤0.05 were considered statistically significant. All statistical calculations were performed using the statistical package R (R Core Team [2018]. R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org).
Results
PATIENTS
Fifty-four consecutive patients underwent coronary IVL in the Royal Victoria Hospital, Belfast, between September 2018 and March 2019. The indication for PCI was chronic stable angina in 46.1% (n=26), non-ST-elevation acute coronary syndrome (NSTEACS) in 33.3% (n=18) and ST-elevation myocardial infarction (STEMI) in 18.5% (n=10) of patients. The majority of patients were in sinus rhythm (n=44). Seven patients were in atrial fibrillation (AF), one patient was in atrial flutter and two patients had a DDD pacemaker in situ.
VENTRICULAR CAPTURE
The incidence of coronary IVL-provoked ventricular capture was 77.8% (n=42). Typical examples of “shocktopics” and runs of asynchronous ventricular pacing are shown in Figure 1. Ventricular capture was associated with a fall in systolic blood pressure (BP) of between 10 and 35 mmHg that immediately resolved on return of intrinsic rhythm. There was no fall in BP if coronary IVL was not associated with ventricular capture.
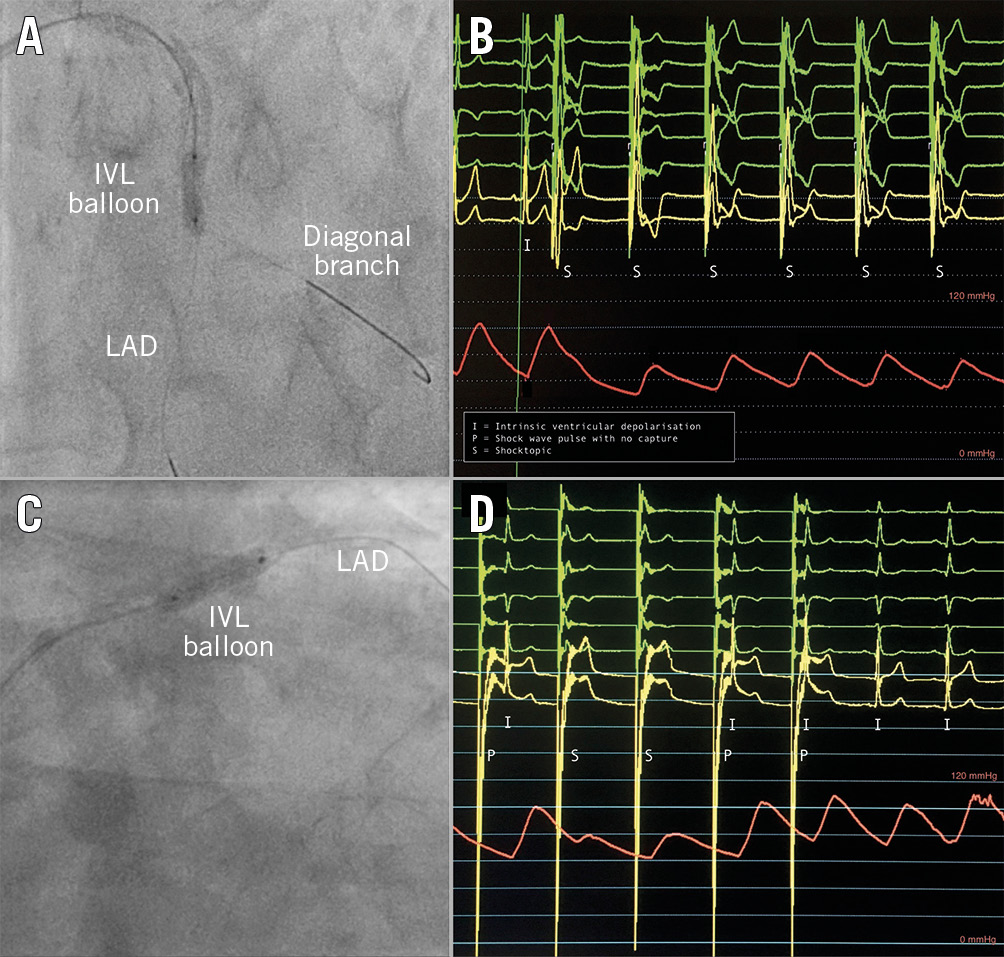
Figure 1. Recordings of IVL-induced cardiac arrhythmia. A) & B) In the first example, the IVL balloon is located within the mid LAD. Shock wave pulses can be seen to precipitate ventricular capture (“shocktopics”) leading to asynchronous ventricular pacing with an associated fall in blood pressure. C) & D) In the second example, the IVL balloon is located within the proximal LAD. The first shock wave pulse does not precipitate a “shocktopic”, but the subsequent two pulses do. Note the large voltage “spikes” that occur with each shock wave pulse.
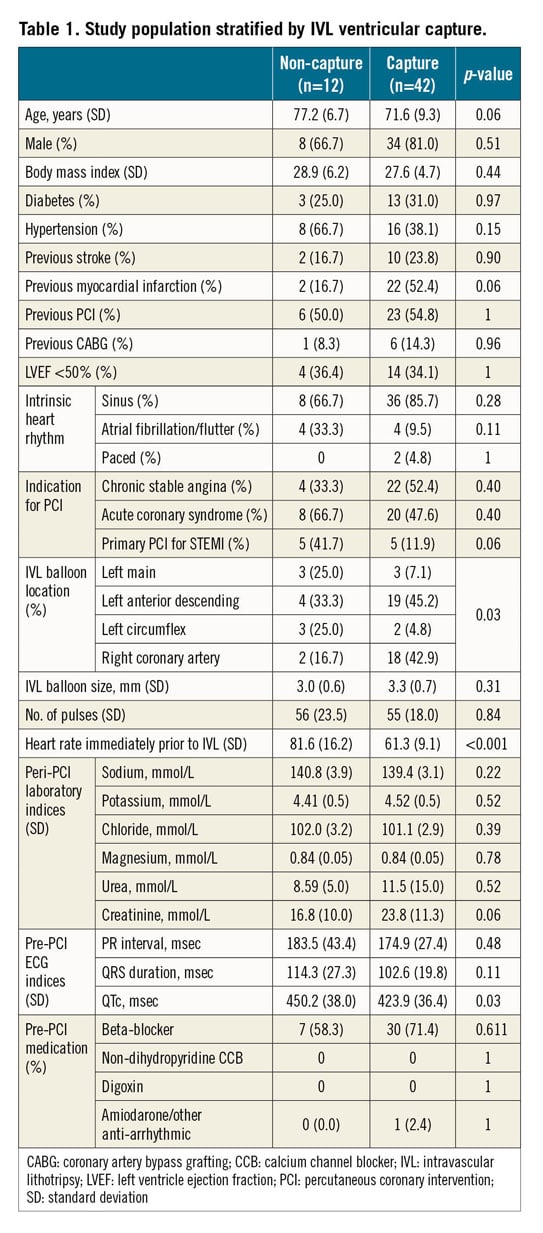
PREDICTORS OF VENTRICULAR CAPTURE
Patient and procedural characteristics are shown in Table 1. Compared to patients who did not experience ventricular capture, patients in whom ventricular capture occurred had a lower intrinsic heart rate (61 vs 82 bpm, p<0.001), were more likely to have had IVL to the left anterior descending (LAD) artery (45.2% vs 33.3%) or right coronary artery (RCA) (42.9% vs 16.7%, p=0.03), and had a shorter QTc interval (424 vs 450 msec, p=0.03). These patients also tended to be younger, have suffered from a previous MI, have a higher creatinine level, and not to be undergoing IVL in the context of primary PCI for STEMI. There were no differences in LV systolic function, periprocedural blood electrolyte concentrations or preprocedural rate-limiting/antiarrhythmic medications.
Multivariable logistic regression analysis identified heart rate as the only independent predictor of an increased risk of IVL-induced ventricular capture. Patients with a heart rate of <65 bpm prior to IVL were over 16 times (odds ratio [OR] 16.3; 95% CI: 2.4-110.8, p=0.004) more likely to experience arrhythmic beats (Figure 2).
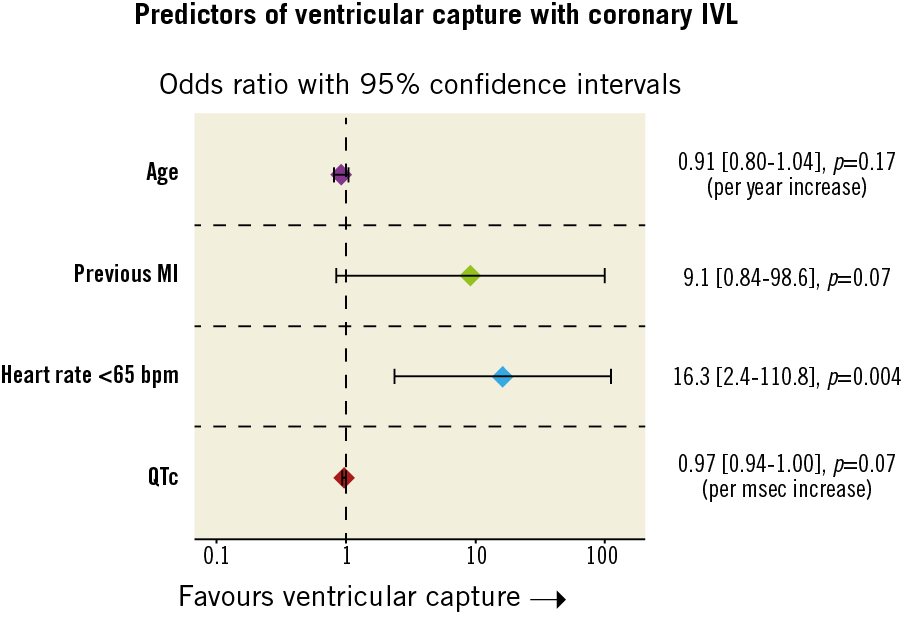
Figure 2. Independent predictors of ventricular capture determined by multivariable logistic regression. Variables for the final model selected by stepwise regression using Akaike information criteria. Candidate variables: age, previous MI, primary PCI, IVL balloon location, resting heart rate ≤65 bpm, creatinine, and QTc.
MULTI-LEAD ECG AND INTRACARDIAC ANALYSES
“Shocktopic” beat morphology was largely uniform in each patient and appeared to be dependent on the target lesion location (Figure 3). Atrial pacing was identified in three patients and in three patients shockwave pulses not associated with ventricular capture were sensed and miscounted by the ECG monitoring as an “R” wave (Figure 4). In our cohort, both patients who had a pacemaker experienced ventricular capture. A post-procedure device check revealed no evidence of pacemaker malfunction. However, to determine if shockwave pulses not associated with capture were (inappropriately) sensed requires live interrogation, as this would not be logged as an event.
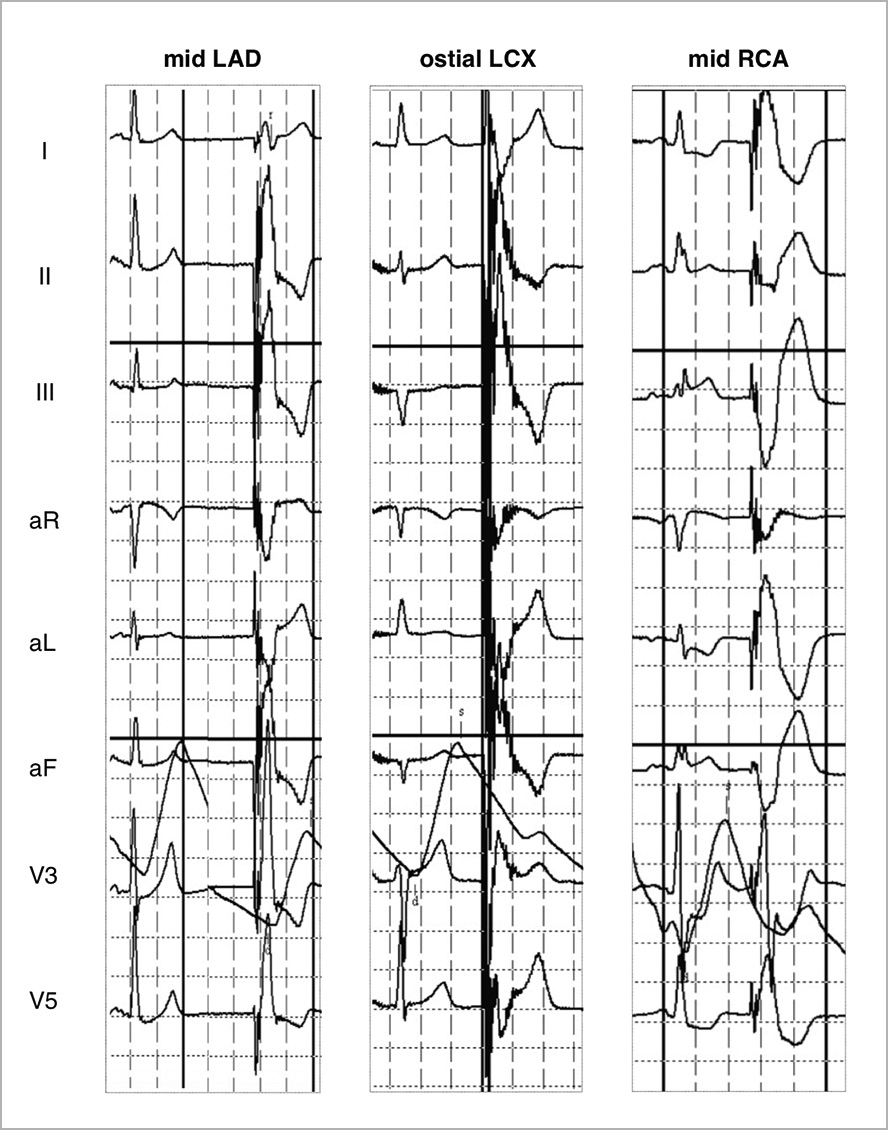
Figure 3. ECG recordings of “shocktopics” in different patients stratified by target vessel. The morphology varied depending on target lesion location with ventricular capture appearing to begin in close proximity to the IVL balloon.
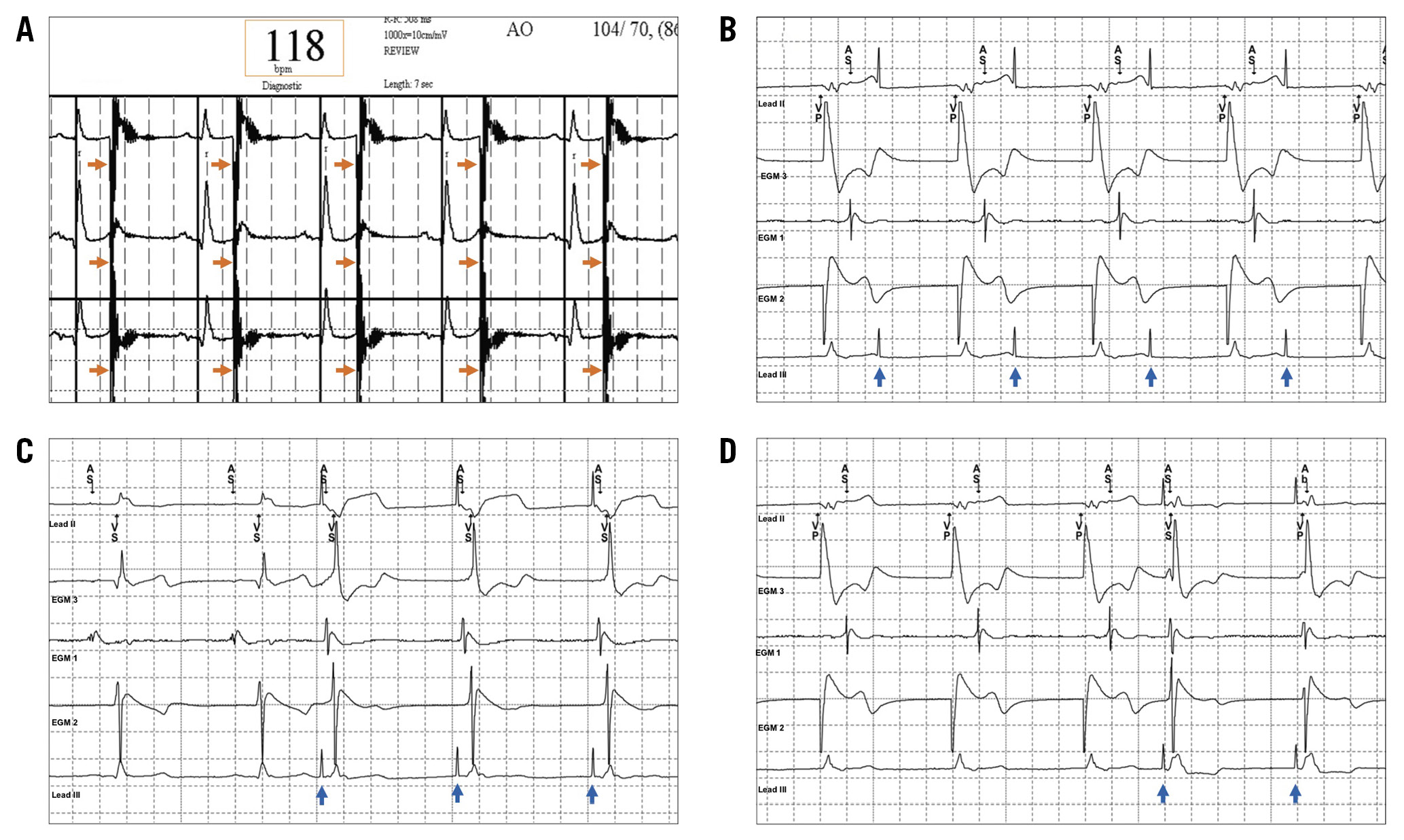
Figure 4. ECG (A) and intracardiac electrogram (B-D) recordings during IVL in two different patients. A) The shock wave pulses (orange arrows) land on the “T” wave of the preceding intrinsic heartbeat but do not trigger ventricular capture/arrhythmia. However, they are interpreted as “R” waves leading to miscalculation of the true heart rate. B) The patient is V-pacing with sensing of retrograde atrial activation. The shock wave pulses (blue arrows) are neither sensed nor associated with capture. In panel C, shock wave pulses (blue arrows) lead to atrial and ventricular capture, whereas in panel D shock wave pulses (blue arrows) lead to simultaneous atrial and ventricular capture.
Figure 4 shows intracardiac electrograms obtained during IVL in a patient with a DDD pacemaker from a collaborative centre (St George’s Hospital, London, United Kingdom). IVL can be seen to result in both atrial and ventricular activation (simultaneous) with appropriate sensing of mechanical capture by the device. There were no instances of shock wave pulses not associated with capture being mis-sensed in either the atrial or ventricular channel.
SAFETY
No adverse clinical events including atrial or ventricular tachyarrhythmia occurred as a result of coronary IVL-induced capture. One patient died in the catheterisation laboratory in the context of a STEMI complicated by cardiogenic shock.
Discussion
The present study highlights that coronary IVL with the Shockwave Medical system, a novel technology for intracoronary calcification modification, is associated with a high incidence of “shocktopics” and asynchronous cardiac pacing. Operators and staff working within the coronary catheterisation environment should be aware of this and the potential associated issues.
Our finding that IVL delivered in close proximity to the myocardium is associated with a high incidence of ventricular capture is not surprising. Lithotripsy-induced cardiac arrhythmia is already well described in the setting of treating renal and ureteric calculi, with a reported incidence as high as 80% using early extracorporeal shock wave lithotripsy (ESWL) devices8,9,10. Ventricular capture was recorded during IVL in all three epicardial vessels although it appeared less common within the left circumflex (LCx) artery. However, the only independent predictor was heart rate. Patients with a heart rate <65 bpm were over 16 times more likely to experience IVL-induced “shocktopics”. It is important to be aware of this as it identifies patients at greatest risk of ventricular capture and therefore any potential complications.
Ventricular capture consistently resulted in a fall in systolic BP of between 10 and 35 mmHg. This was not due to balloon-induced myocardial ischaemia as there was no change in BP in the absence of capture. Instead, these findings can be explained by incomplete ventricular filling and/or dyssynchronous ventricular depolarisation that is typical of ectopic beats. Blood pressures reverted to pre-treatment levels on return of intrinsic rhythm, indicating that the likelihood of adverse sequelae is remote.
Coronary IVL does not emit an electrical current outwith the transducer. Instead, atrial or ventricular capture is the result of mechano-electric coupling between the energy from the sonic pressure waves and the cardiac conduction system11. This is analogous to percussion pacing or ectopics induced during cardiac chamber instrumentation12,13. Previous studies have shown that myocardial depolarisation in response to regional mechanical stimulation is primarily due to activation of local stretch-activated cardiomyocyte channels14,15,16,17. Consistent with this as the basis for coronary IVL cardiac pacing, “shocktopic” complex morphology was dependent on target lesion location with ventricular capture appearing to begin in close proximity to the emitter source.
Our findings raise a number of potential issues. While atrial capture is unlikely to be of consequence, “shocktopics” could in theory provoke VT/VF given the capacity for asynchronous “R on T” pacing. Numerous studies have shown that VT/VF can reliably be induced by a mechanical impulse (e.g., commotio cordis)17,18,19,20,21. However, as this is critically dependent on the impact landing precisely over the trailing edge of the preceding ventricle repolarisation wave, the vulnerable window is narrow in both time and location. Other factors also suggest that the probability of VT/VF induction is likely to be low. Ventricular capture with coronary IVL is comparable to VOO pacing in that both are asynchronous and lack the ability to sense intrinsic cardiac activity. Historic data indicate that VOO pacing is associated with a low risk of significant arrhythmia and, although much less used in contemporary practice, transient mode switching to VOO remains commonplace during interrogation of modern devices22.
On the basis of animal data, historic pacemaker data, and the absence of VT/VF generation in the present study (albeit in a limited sample size), the risk of coronary IVL precipitating VT/VF would appear to be low. However, this remains to be fully defined and may be higher compared to other mechanical stimuli of similar magnitude considering the target population and the effects of acute (e.g., as a consequence of coronary IVL balloon inflation or in the context of acute coronary syndrome) and chronic myocardial ischaemia on arrhythmogenic susceptibility16,17. ECG gating would in theory remove this risk entirely and is incorporated (although not used as standard) into most modern ESWL devices20,21,22. These devices, however, deliver a substantially greater number of pulses at a similar or higher frequency23.
The Shockwave Medical coronary IVL system utilises spark-gap technology (electrohydraulically generated lithotripsy) to generate the shock wave. Thus, while any electrical current is isolated from the myocardium and “effectively contained” within the IVL balloon, an EM signal is invariably produced. These EM signals account for the voltage “spikes” observed on ECG monitoring with each coronary IVL pulse, that in some cases were misinterpreted as intrinsic “R” waves. Similar mis-sensing of EM signals by a pacemaker could lead to inappropriate device inhibition. In pacemaker-dependent patients, this has the potential for loss of output for up to 10 secs (maximum duration of treatment) if unrecognised and the IVL pulse is not associated with ventricular capture. However, it is important to emphasise that we have not observed such an issue and, in the absence of reports of pacemaker mis-sensing, this remains unsubstantiated. Moreover, in the one patient so far to have live device interrogation, IVL pulses not associated with mechanical capture did not result in mis-sensing in either the atrial or ventricular channel. This may reflect more advanced EM bandpass filters in these devices. The effects of coronary IVL on implanted devices will be systematically evaluated in the DISRUPT CAD III study (NCT03595176).
Limitations
This report highlights the incidence of coronary IVL-induced “shocktopics” and cardiac pacing at a single centre, potentially limiting extrapolation of our findings. However, patient and procedural characteristics were unremarkable with events recorded by seven different operators. Furthermore, similar experiences have been observed in three other contributing centres (St George’s Hospital, London, United Kingdom; King’s College Hospital, London, United Kingdom; and Bristol Heart Institute, Bristol, United Kingdom). The risk of coronary IVL provoking VT/VF is largely extrapolated from animal studies of commotio cordis and historic data from pacemaker interrogations. More robust assessment of the electrophysiological phenomena associated with coronary IVL is required and will be provided in a number of planned substudies of the DISRUPT CAD III trial.
Conclusions
Coronary IVL with the Shockwave Medical coronary IVL system is associated with a high incidence of “shocktopics” and asynchronous cardiac pacing that is largely dependent on the resting heart rate. There were no clinical events associated with this phenomenon, but further systematic evaluation is warranted.
|
Impact on daily practice This study highlights that coronary IVL with the Shockwave Medical coronary IVL system is associated with a high incidence (77.8%) of ventricular ectopics (“shocktopics”) and asynchronous cardiac pacing. These events occur as a result of mechano-electrical coupling and are strongly dependent on the resting heart rate. Patients with a heart rate <65 bpm prior to IVL were sixteen-fold more likely to experience ventricular capture compared to patients with a heart rate ≥65 bpm. While there have been no clinical events associated with this phenomenon to date, operators and staff working within the coronary catheterisation environment should be aware of this and the potential associated issues. |
Acknowledgements
The authors would like to thank Uday Illindala (PhD), employee of Shockwave Medical and Todd Brinton (MD), co-founder of Shockwave Medical for their assistance. In addition, we thank Dr Andrew McNeice, Dr Paul Johnston, Dr James Shand, Dr Tom Johnson, Dr Julian Yeoh, Dr Ibrahim Yearoo, Dr Anenta Ramakrishnan, and Dr Colum Owens for their contribution.
Conflict of interest statement
J. Hill has received research support and honoraria from Shockwave Medical. H. Halperin has received consulting fees from Shockwave Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

