Abstract
Aims: To investigate in vivo relationships between segmental wall shear stress (WSS), endothelium-dependent vasoreactivity and arterial remodelling.
Methods and results: Twenty-four patients with minor angiographic coronary arterial disease (≤30% stenosis severity) underwent intracoronary (IC) salbutamol provocation during intravascular ultrasound (IVUS)-upon-Doppler guidewire imaging. Macrovascular response (change in segmental lumen volume [SLV] at baseline and following IC salbutamol), plaque burden (percent atheroma volume [PAV]), remodelling indices (RI), eccentricity indices (EI) and WSS were evaluated in 179 consecutive 5 mm coronary segments. Baseline WSS was directly related to endothelium-dependent epicardial coronary vasomotion (% change SLV, coefficient 17.2, p=0.004), and inversely related to RI (coefficient –0.23, p=0.02) and EI (coefficient –10.0, p=0.001). Baseline WSS was lower in segments displaying endothelial dysfunction (defined as any change in SLV ≤0) compared with preserved function (0.66±0.33 vs. 0.71±0.22 N/m2, p=0.046). Independent of plaque burden, segments with the lowest tertile of WSS displayed less vasodilatation, or vasoconstriction, than segments with the highest tertile of WSS. Higher plaque burden segments harbouring the lowest tertiles of WSS displayed vasoconstriction, expansive arterial remodelling and greater plaque eccentricity.
Conclusions: In patients with stable coronary syndromes and minor angiographic coronary disease, coronary segments with lower in vivo WSS values display functional and morphological features of plaque vulnerability.
Introduction
While atherosclerosis is a diffuse, systemic process, its consequences are typically encountered in a focal, segmental manner. Although the earliest manifestation of the disease process is characterised by dysfunctional endothelium, local impairment in endothelial homeostatic mechanisms may promote plaque progression, vasoconstriction and superimposed thrombus formation in the setting of established atherosclerosis, leading to a clinical event1.
Regional wall shear stress (WSS) is thought to influence endothelial phenotype, as well as the development and progression of coronary atherosclerosis2,3. Prior studies have shown that baseline in vivo coronary WSS patterns are associated with differential effects on plaque burden, composition and associated vascular remodelling in humans4,5. Both the baseline burden of coronary atherosclerosis6-8 and coronary endothelial function9-11 have been shown to be associated with future coronary events. However, to date, no published human in vivo study has directly explored the association between coronary WSS patterns, plaque burden and arterial remodelling with corresponding segmental endothelium-dependent vasomotor function. Utilising intravascular ultrasound (IVUS) and Doppler guidewire imaging during intracoronary (IC) provocation, we have previously shown that IC salbutamol is an endothelium-dependent vasomotor stimulus, exhibiting nitric oxide (NO)-dependent properties within the human epicardial coronary system and the coronary microvasculature in vivo12. The current study investigated the relationship between baseline WSS patterns and segmental coronary vasoreactivity in regions with varying plaque burden.
Methods
STUDY SUBJECTS
We enrolled twenty-four consecutive patients (aged ≥18 years) referred for coronary angiography for the investigation of chest pain, or chest pain equivalent syndrome. After the patients had provided informed consent, all vasoactive medications were held for at least 24 hrs prior to the study. All procedures were performed in the morning after an overnight fast. Excluded were patients with significant “angiographic” coronary artery disease, significant valvular heart disease, left ventricular dysfunction, prior percutaneous or surgical revascularisation, acute coronary syndrome (defined by electrocardiography changes and/or troponin elevation) within the last four weeks, known coronary spasm, severe obstructive lung disease, creatinine clearance ≤60 mL/min or the use of short or long-acting β2 agonists within the previous 12 hrs. Target vessels with visual angiographic stenoses of >30% were excluded, as were those that included a significant branch vessel (≥2 mm diameter) within the area to be studied. This study was approved by the Royal Adelaide Hospital Human Research Ethics Committee.
CARDIAC CATHETERISATION AND INTRAVASCULAR IMAGING PROTOCOLS
Coronary angiography was performed via a standard 6 Fr technique. Intravenous heparin (70 IU/kg) was administered for the research protocol. A 0.014 inch Doppler guidewire (Flowire; Volcano Therapeutics, Rancho Cordova, CA, USA) was placed into the target vessel within its mid segment away from major side branches. This wire was also used to deliver a 2.5 Fr, 40 MHz Atlantic Pro IVUS catheter (Boston Scientific, Natick, MA, USA) into the study artery. This was undertaken without pretreatment with IC nitroglycerine (NTG). All IC infusions were administered through a Medrad infusion pump at 2 mL/min via the coronary guiding catheter for a period of five minutes. Following three minutes of IC infusion (of either vehicle solution or salbutamol) and acquisition of a stable Doppler guidewire signal, the instantaneous average peak velocity (APV) signal was recorded, followed by the Doppler guidewire being repositioned into the distal vessel for the remaining two minutes. The IVUS catheter was then moved from within the guiding catheter into the distal conduit vessel and images were acquired during automated catheter withdrawal at 0.5 mm/sec. Prior studies have shown that repeated, consecutive IC vehicle infusions every five minutes during IC instrumentation with an IVUS-upon-Doppler guidewire imaging protocol have no significant impact on changes in vascular measurements over time12. The IVUS images were recorded on a DVD for off-line analysis.
CORONARY INFUSION AND ENDOTHELIAL FUNCTION TESTING PROTOCOLS
The active infusion protocols that were performed as part of the validation of IC salbutamol as an endothelium-dependent coronary vasomotor stimulus have been previously described in detail12. Briefly, an IC salbutamol dose-response infusion protocol (0.15 μg/min, 0.30 µg/min, 0.60 µg/min) was initially performed to confirm its vasoactive effects and to select the optimal dose for assessment of its NO-dependent properties. The 0.30 µg/min salbutamol dose was chosen as the optimal dose to infuse both prior to and following the infusion of IC NG-monomethyl-L-arginine (L-NMMA), confirming its NO-dependent properties. Therefore, for the purposes of analysing the relationships between baseline segmental WSS patterns and endothelium-dependent vasomotor reactivity, all haemodynamic and IVUS measurements were taken at baseline and following the administration of a 0.30 μg/min dose of salbutamol, a validated endothelium-dependent vasomotor stimulus in both the human epicardial and coronary microvasculature (Figure 1)12,13.
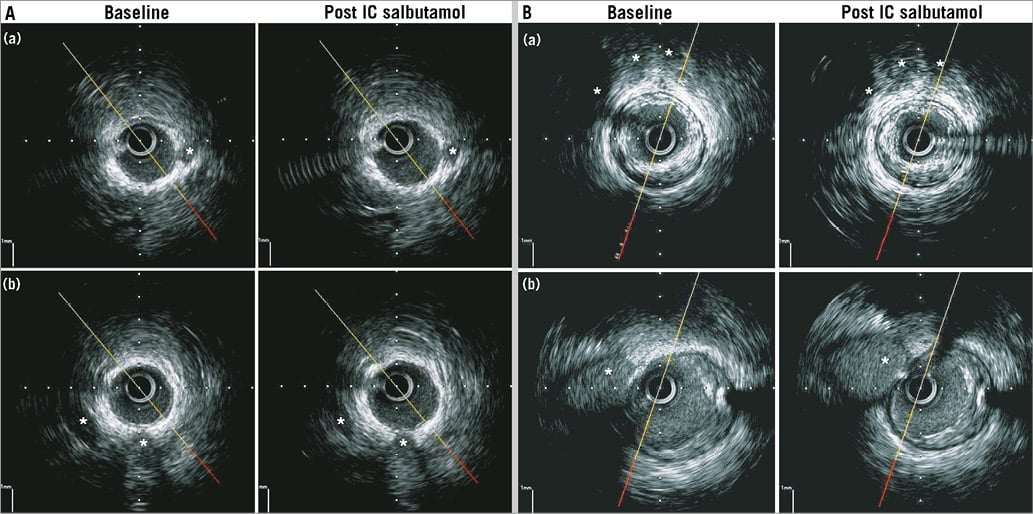
Figure 1. Endothelium-dependent coronary vasomotion in low and high plaque burden segments. A) Two examples of where a representative frame within a low plaque burden segment underwent vasodilation following IC salbutamol. In segment (a), PAV was 10.5%. This segment underwent 21.1% increase in SLV from baseline. In segment (b), PAV was 15.4%. This segment underwent 26.2% increase in SLV from baseline. * represent fiduciary markers (side branches or coronary veins). B) Two examples of where a representative frame within a low plaque burden segment underwent vasoconstriction following IC salbutamol. In segment (a), PAV was 48.0%. This segment underwent 31.4% decrease in SLV from baseline. In segment (b), PAV was 38%. This segment underwent 22% decrease in SLV from baseline. * represent fiduciary markers (side branches or coronary veins).
DATA ACQUISITION AND ANALYSIS
All IVUS data were analysed using echoPlaque 3.0.53 (INDEC Medical Systems, Santa Clara, CA, USA). Proximal and distal fiduciary markers (anatomical side branches) were chosen to define the overall region of the vessel to be analysed, as well as for segment matching. Cross-sectional images were selected every 30 frames (0.5 mm) apart. Frames that precluded complete lumen or vessel wall planimetry were excluded from analysis, as were segments that involved bifurcating branch points. Each IVUS pullback was divided into contiguous, non-overlapping 5 mm segments comprising 11 frames taken at 10 evenly spaced cross-sectional (0.5 mm) intervals. Given the known segmental heterogeneity of coronary vasomotor reactivity14-16, which we previously showed was predominantly influenced by the regional variation in corresponding IVUS-derived plaque burden, each segment was thus analysed separately as an individual entity12. Using MIB software (INDEC Medical Systems), the matching frames of anatomical side branches from the baseline and post-salbutamol stimulation IVUS runs were co-registered to ensure accurate segment matching between runs. Leading edges of the lumen and external elastic membrane were manually planimetered. Percent atheroma volume (PAV) was calculated to determine segmental plaque burden6,12:
PAV=∑ [(EEMarea – Lumenarea) / ∑ EEMarea]×100
Lower and higher plaque burdens were divided around the mean PAV of all segments. Segmental lumen volumes (SLV) were calculated as the summation of lumen area in each measured image. As some frames were technically inadequate for complete IVUS analysis, the SLV for each 5 mm segment was normalised to account for differences in the number of analysable frames within each predefined segment, as previously described12:
SLVnormalised=∑ [(Lumenarea)/number of images in segment]×10
Segmental remodelling and eccentricity indices were also calculated, as previously described12,17. Briefly, segmental remodelling indices (RI) were determined by calculating the average segmental EEMarea and dividing this by a reference EEMarea taken from either a proximal or a distal reference point located within 10 mm from the index segment with the least plaque burden, or before a major branch point. Segmental eccentricity indices (EI) were determined by calculating the average of all EIs of each analysable frame within a coronary segment (EI=ratio of maximal to minimal plaque thickness). All measurements were performed by a single analyst blinded to the specific segment and degree of vasomotor response to IC salbutamol in the preceding infusion. Intra- and inter-observer variability analysis was performed following planimetry of lumen and plaque areas from 20 randomly selected IVUS frames by two independent observers and by one observer at two time points separated by one week.
A Doppler guidewire-derived APV was determined from instantaneous velocity signals from the wire by an online fast Fourier transform. Wall shear stress within each 5 mm segment was calculated assuming Poiseuille flow yielding the equation: WSS=8ην/d, where η is blood viscosity (assumed to be constant at 3.5 mPa·s, and unaltered by intracoronary infusions), ν represents the average cross-sectional velocity at the position of the Doppler wire, and d corresponds to the average diameter of the 5 mm coronary segment measured from each of the selected IVUS frames analysed for that segment. As the assumption of a parabolic Poiseuille flow in a circular straight tube (corresponding to each 5 mm segment) was made, v was taken as half the measured APV18-20. This method of WSS calculation is fundamentally the same as originally used by Wentzel et al21, Gijsen et al22, Doriot et al23 and more recently by van’t Veer et al19. However, we did not use a finite element model to calculate segmental WSS, which should be regarded as “gold standard”. The formula described above is the analytic solution of the Navier-Stokes equations, under the assumptions of an incompressible, steady, laminar Newtonian blood flow19,23. This simpler assumption of a Newtonian model of flow has been shown to yield equivalent WSS values to those obtained during non-Newtonian flow modelling, and can be viewed as a complementary technique of assessing WSS in vivo24.
Statistical analysis
Data are expressed as mean±SD or median (interquartile range) when data were non-normally distributed. Mixed effects models were employed for examining the relationship between continuous variables, with subject identity and segment number modelled as random effects, where appropriate, to account for repeated measures within a given segment in a particular individual. Baseline WSS was modelled as the predictor variable (both as a continuous variable and as a categorical variable in tertiles) against various continuous variable parameters of coronary arterial morphology and endothelial function as dependent variables (which were transformed where appropriate). To evaluate whether PAV might have a confounding effect on these various dependent variables, PAV was included in the mixed effects model as a binary covariate (PAV ≤ or > the mean of 27.3%) in an interaction term with WSS. If this interaction term proved non-significant, it was removed from the model, and PAV and WSS entered as separate and independent predictor variables. A similar approach was used to examine possible confounding effects of smoking, diabetes and hypertension on outcome variables of interest. For the purposes of sub-analysis, we divided the range of segmental WSS values obtained in our study into tertiles. All statistical tests were two-sided and a p-value <0.05 was considered significant. Statistical analysis was performed with Stata 11 (StataCorp, College Station, TX, USA).
Results
PATIENT DEMOGRAPHICS, HAEMODYNAMICS AND OBSERVER VARIABILITY DATA
Baseline demographics of the study cohort are shown in Table 1. Of the 24 coronary arteries interrogated, 21 (88%) involved the left anterior descending artery, two (8%) involved the circumflex artery, and one (4%) the right coronary artery. Overall, 1,005 mm of vessel (2,010 cross-sectional frames on IVUS, equating to 201 contiguous, non-overlapping 5 mm segments) were analysed in 24 individuals. Of these 201 contiguous 5 mm segments, 22 segments crossed side branches and were thus excluded from analysis, leaving a total of 179 contiguous 5 mm segments for analysis, which had a mean lumen diameter of 3.4 mm. There were no significant changes in baseline heart rate or blood pressure following IC salbutamol infusion. For coronary lumen measurements, the intra-observer coefficient of variation was 1.1%, and the inter-observer coefficient of variation was 2.6%. For plaque measurements, the intra-observer coefficient of variation was 1.8%, and the inter-observer coefficient of variation was 3.8%.
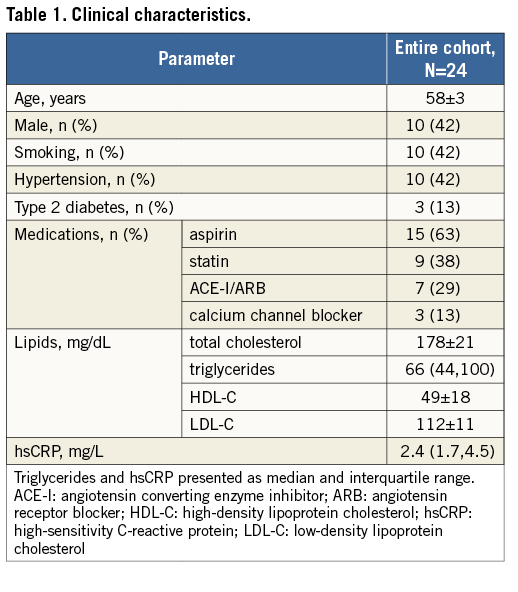
RELATIONSHIPS BETWEEN WALL SHEAR STRESS, ENDOTHELIAL FUNCTION AND ARTERIAL MORPHOLOGY
Table 2 highlights the associations between baseline WSS, segmental coronary endothelial function and plaque geometry. The baseline WSS of all 179 coronary segments was 0.68±0.27 N/m2 (range of WSS from 0.18 N/m2 to 1.56 N/m2). There was a significant positive association between baseline segmental WSS and the percent change in SLV (endothelium-dependent coronary vasomotor reactivity). Baseline WSS was negatively associated with the remodelling index (RI) and the segmental eccentricity index (EI). Table 3 further describes these relationships when coronary segments are stratified according to tertiles of baseline WSS, and when adjusted for factors possibly influencing endothelial function, which included smoking, hypertension and diabetes mellitus. This table highlights that, in segments containing the lowest tertile of WSS, vasomotor reactivity was significantly impaired coupled with greater plaque eccentricity, compared to the highest tertile of WSS. Further analysis revealed that, irrespective of plaque burden (divided according to the mean PAV of all segments), baseline WSS imparted a significant influence upon coronary vasomotor reactivity (the mixed model interaction between plaque burden [PAV] and WSS was not significant, and the p-value for the interaction between WSS tertiles 1 and 3 and the percent change in SLV was 0.01) (Figure 2).
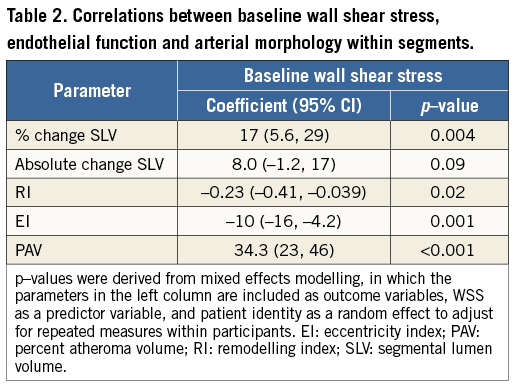
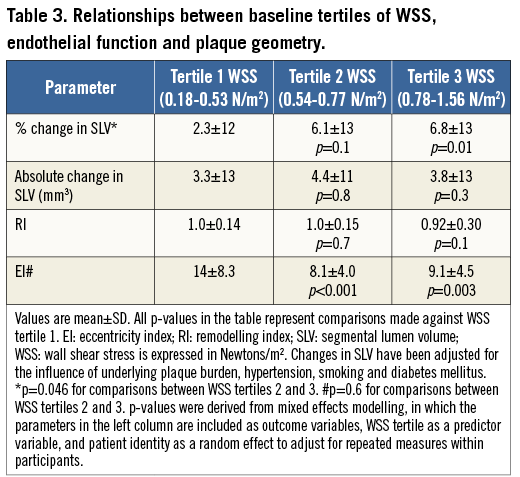
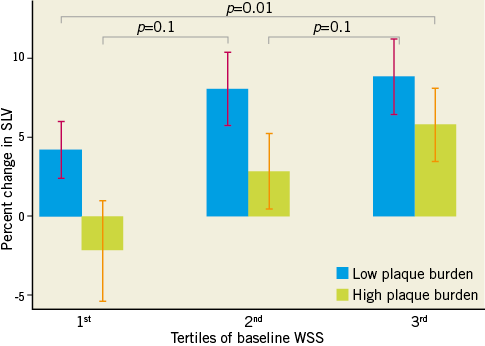
Figure 2. Relationship between coronary endothelial function and tertiles of WSS, stratified for plaque burden. There was a significant relationship between the tertile of segmental WSS and the corresponding percent change in SLV (endothelial function). The interaction between WSS tertile and plaque burden (PAV group) was not significant. Compared to the highest tertile of WSS, the lowest tertile of WSS had significantly reduced endothelium-dependent coronary vasomotion. p-values reflect between-tertile comparisons.
The mean plaque burden of all 179 coronary segments was 27.3% (range of PAV from 6.0% to 64.7%). In the group of segments containing plaque burden below the mean (with a mean PAV 15.9%), the baseline SLV was 97.0 mm3. The baseline SLV of segments with plaque burden above the mean (with a mean PAV 39.9%) was 76.8 mm3 (p<0.001 compared with lower plaque burden group). Overall, conduit segment endothelium-dependent vasomotor reactivity was significantly greater in segments with plaque burden below the mean than in segments with PAV above the mean (percent change in SLV 6.76±12.0 vs. 2.39±13.1%, p=0.02; absolute change in SLV 5.92±11.0 vs. 1.31±9.68 mm3, p=0.002). There were no significant differences in RI (0.99±0.01 vs. 1.00±0.28, p=0.6) or EI (9.05 [6.67, 12.8] vs. 9.35 [5.98, 16.0], p=0.8) in those segments with lower or higher PAV, respectively.
Normal endothelial function was defined as any increase in SLV9,11. Accordingly, in segments displaying normal endothelial function, baseline WSS was higher (0.71±0.22 N/m2) compared to segments exhibiting endothelial dysfunction (0.66±0.33 N/m2, p=0.046).
WALL SHEAR STRESS, PLAQUE GEOMETRY AND CORONARY VASOMOTOR REACTIVITY IN HIGHER PLAQUE BURDEN SEGMENTS
Increasing degrees of plaque burden and expansive arterial remodelling are both known to be associated with the vulnerable plaque phenotype1,6,25. As such, we specifically undertook analysis of segments containing greater than the mean plaque burden, to investigate for associations between WSS, plaque geometry and endothelial-dependent vasomotion. Vasoconstriction was observed in segments with higher plaque burden (PAV >mean) that were categorised as having the lowest tertile of WSS (Figure 1). Additionally, in this higher plaque burden group, expansive coronary arterial remodelling (RI >1.05) (Figure 3) and greater plaque eccentricity (Figure 4) were evident in segments harbouring the lowest tertile of WSS.
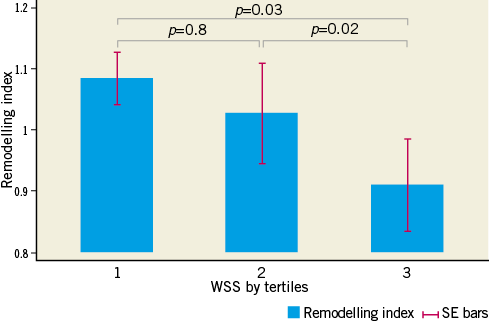
Figure 3. Relationship between remodelling indices and tertiles of WSS, in the higher PAV group. In the higher PAV group, segments harbouring the lowest tertile of WSS displayed expansive coronary arterial remodelling (RI >1.05). This was significantly greater than segments harbouring the highest tertile of WSS, which displayed constrictive arterial remodelling (RI <0.95).
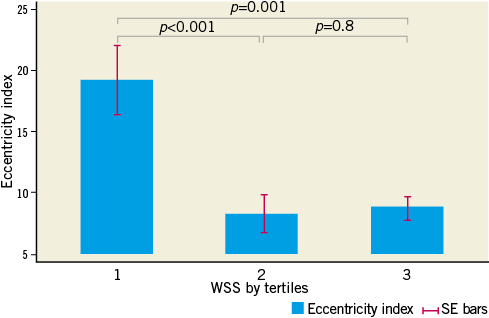
Figure 4. Relationship between eccentricity indices and tertiles of WSS in the higher PAV group. In the higher PAV group, segments harbouring the lowest tertile of WSS displayed significantly greater plaque eccentricity than remaining coronary segments.
Discussion
Interactions between the development of coronary atherosclerosis and subsequent vascular responses are highly complex and poorly understood in humans in vivo. Prior animal26 and human4,5 evaluations of in vivo WSS patterns have suggested local manifestations of disease phenotype, in line with previous observations of localised vascular endothelial behaviour27. Our study is the first to show a significant, direct relationship between coronary endothelium-dependent vasomotor reactivity with corresponding baseline WSS patterns in humans in vivo. Our observations extend previous cell culture experimental findings, to confirm impaired NO bioavailability and subsequent coronary vasomotion within regions of low WSS. We show that, irrespective of the underlying burden of coronary atherosclerosis quantified by IVUS, coronary segments with low WSS patterns exhibit reduced endothelium-dependent function. We further show an inverse relationship between WSS patterns and segmental RIs, whereby in segments with low WSS expansive arterial remodelling was found. Similarly, in segments with low WSS, the most eccentric plaque was observed. The observation of direct associations between low WSS, vasoconstriction, expansive remodelling and eccentric plaque distribution evident in higher plaque burden segments highlights for the first time the link between low WSS and contemporary views of vulnerable plaque phenotype in vivo.
Interactions between WSS and the endothelium are essential for the regulation of homeostatic and adaptive arterial mechanisms. Endothelial cell surfaces mediate signal mechanotransduction and subsequent phosphorylation of transcription factors, which ultimately result in gene splicing and alterations of endothelial cell function and morphology2. Wall shear stress is the most potent stimulus for endothelial-derived NO production2,28. Regions of lower WSS alter endothelial cell phenotype, characterised most notably by a reduction in the bioavailability of NO, and thus significant attenuation of NO-dependent vasomotion29. Although there exist a number of experiments evaluating the role of WSS mediating endothelial phenotype, most of these have involved cell culture techniques from a variety of species29,30. As such, there is a paucity of experiments investigating these mechanisms in vivo, particularly in humans.
Coronary plaque progression and instability have been associated with expansive remodelling of the arterial wall25. Although the pathophysiologic mechanisms of coronary arterial remodelling remain unknown, it is likely that regional WSS plays an important role, particularly in the development of expansive coronary remodelling. It is currently believed that low WSS may lead to focal, eccentric plaque development, further propagating a low WSS environment2. Our findings of a general inverse association between segmental WSS patterns, RIs and EIs, in particular the specific associations between lower values of WSS, expansive coronary remodelling and greater plaque eccentricity in segments with higher plaque burden, are in accordance with prior findings in a serial study of atherosclerosis progression in an in vivo swine model26. A more recent magnetic resonance imaging evaluation of rabbit aortic atherosclerosis in vivo revealed lower WSS regions more commonly associated with higher plaque burden, positive vascular remodelling and plaque disruption31. However, it remains unknown what factors determine whether the expansive remodelling process to plaque formation is compensatory or excessive.
Although finite element models of WSS computations (vascular profiling) have been conducted to evaluate influences of static and serial WSS patterns in vivo3-5,32,33, and are now considered “gold standard”, this technique is also not without certain assumptions and limitations23,34. Moreover, our study is the first to administer an IC endothelium-dependent stimulus during subsequent intravascular imaging to evaluate the underlying relationships between plaque burden, vasomotor reactivity, remodelling and WSS patterns across whole, contiguous, non-overlapping human coronary segments in vivo. From a methodological perspective, the unique aspect of this study was that patients underwent continuous, invasive IVUS-upon-Doppler guidewire imaging during IC provocation. While limitations exist regarding our methodology, particularly regarding assessment of WSS, a particular focus regarding the design of this study was to assess the “most robust” yet simplest, single imaging modality to evaluate “high-risk” vessel characteristics. This removes the significant issue of the need for multimodality image co-registration and detailed, complex off-line engineering analysis and image reconstruction, which itself introduces a number of sources of error, and renders the process of in vivo WSS characterisation arduous and expensive with limited practicality.
Acknowledging certain methodological strengths, several additional caveats, however, need to be considered with regard to the interpretation of these findings. In our study, the associations between WSS, endothelial and plaque/vessel phenotype pertain to a single time point, and cannot be extrapolated to temporal associations between WSS and plaque morphology. The validation of IC salbutamol as an endothelium-dependent stimulus using an IVUS-upon-Doppler Flowire technique during IC provocation was highly invasive and not previously reported in vivo in humans. This involved IC infusions of vasoconstricting substances in vessels not pretreated with IC NTG. As such, we evaluated patients with stable, minor angiographic coronary artery disease and hence our findings cannot be extrapolated to unstable patients with obstructive coronary arterial lesions. Nevertheless, our WSS measurements are perhaps the only human in vivo measurements made in the coronary circulation without the pretreatment of IC NTG, and thus they are more likely to represent true in vivo biological values. Direct vasodilator responses to IC NTG injection were not evaluated. In theory, this would have allowed us to test whether smooth muscle cell dysfunction, rather than impairment in NO-dependent function, contributed to blunted vessel wall reactivity in a number of segments: this mechanistic information would have added significance to our observations. Relationships between WSS and plaque composition were also not evaluated. Although the subject of recent evaluation5, plaque compositional analysis with IVUS is not without its limitations and itself warrants further evaluation. As previously mentioned, formal vascular profiling, involving centreline reconstruction of the IVUS images upon 3D coronary angiography reconstructions, and then finite element analysis and computational fluid dynamics, is the current “gold standard” approach for in vivo WSS profiling, and the lack of this approach in our experiments remains a fundamental limitation. We, however, adopted a far more simplistic, limited approach, based on a number of physical assumptions used by previous investigators. As a result, our WSS results should be interpreted with a degree of caution. We also obtained a single average WSS value per 5 mm non-bifurcating coronary segment, which is a diluted, perhaps surrogate estimate of the true, multiple focal WSS profiles probably found in curved, complex structures analogous to the coronary tree. The use of the Poiseuille model does not allow for the evaluation of a number of flow characteristics that occur in pulsatile, curved structures, such as the epicardial coronary vasculature. A single velocity profile was obtained from positioning the Doppler Flowire in the centre of the entire imaged 30-40 mm coronary segment away from side branches, rather than direct ascertainment of segmental flow patterns. Although velocity profiles within arteries are often non-parabolic (e.g., in curved segments and at bifurcations), the Poiseuille model has been previously used, as it was considered to allow a reasonable approximation of the assessments of mean WSS in small vessels. According to Benson et al35, the effects of pulsatility and other non-ideal aspects are able to be neglected for small-calibre vessels perfused by low flow, as is the case within the human coronary vasculature23. As previously mentioned, the strength of our approach was the utility of IVUS for all data points assessed, therefore negating the issues of image co-registration of multiple imaging modalities to arrive at our conclusions.
Conclusions
In stable patients with minor angiographic coronary lesions, segmental WSS is directly related to the endothelium NO-dependent vasomotor response, and inversely related to coronary arterial remodelling and plaque eccentricity indices. Independent of the burden of atherosclerosis determined by IVUS, regions with lower WSS are associated with more impaired NO-dependent vasomotor reactivity. Higher plaque burden segments with low regional WSS are associated with vasoconstriction, expansive remodelling and greater plaque eccentricity. These findings shed further light on associations between factors implicated in coronary atherogenesis and plaque vulnerability. A greater understanding of these mechanisms may ultimately allow for the development of novel risk-prediction algorithms for the evaluation of the natural history of human coronary atherosclerosis in vivo.
| Impact on daily practice Physiological wall shear stress (WSS) is fundamental for regulating normal endothelial homeostasis, yet direct human observations of this relationship in vivo have proven elusive. Our demonstration of low regional WSS associating with segmental coronary endothelial dysfunction, expansive arterial remodelling and eccentric plaque distribution in high plaque burden segments, without the need for formal vascular profiling and off-line computational modelling, highlights the strength of these physiological associations in vivo. This more simplistic approach of WSS estimation could ultimately enable clinicians to identify coronary segments at greater risk for causing future coronary events in the cathlab more readily. |
Acknowledgements
We would like to acknowledge the nursing, technical and radiography staff of the Cardiovascular Investigation Unit of the Royal Adelaide Hospital. We would also like to acknowledge Drs D.G. Katritsis and J. Pantos of the Athens Euroclinic, Athens, Greece, for their technical advice.
Funding
R. Puri, D. Leong and G. Liew are individually supported by a Postgraduate Medical Research Scholarship from the National Health & Medical Research Council (565579, 519177, and 497809, respectively). R. Puri and D. Leong are jointly funded by the National Heart Foundation of Australia (PC0804045, PC07A3395) and Dawes Scholarships (Hanson Institute). Equipment funding for this study was obtained from a Cardiovascular Lipid Grant, Pfizer Australia Pty Ltd (CB21.08). M. Worthley is a SA Health Early to Mid Career Practitioner Fellow.
Conflict of interest statement
The authors have no conflicts of interest to declare.

