Abstract
Aims: The association of frailty with coronary plaque phenotype among older patients with non-ST-elevation acute coronary syndrome (NSTEACS) is not known. The aim of this study was to evaluate the association of frailty with coronary plaque phenotype among older patients with NSTEACS.
Methods and results: Older patients with NSTEACS who underwent invasive angiography were recruited. Frailty was measured using the Fried frailty score. Following angiography, patients underwent greyscale and virtual histology intravascular ultrasound (VH-IVUS) imaging. Of the 90 patients, 26 (28.9%) were robust, 49 (54.4%) patients were pre-frail, and 15 (16.7%) were frail. Mean age was 80.9±3.8 years; 59 (65.6%) were male. Compared to robust patients, the pre-frail group had a significantly greater presence of high-risk lesions including VH thin-cap fibroatheroma (TCFA, p=0.011), minimum lumen area (MLA) ≤4 mm2 (p=0.016), TCFA+MLA ≤4 mm2 (p=0.005), TCFA+plaque burden (PB) ≥70% (p=0.005) and TCFA+PB ≥70%+MLA ≤4 mm2 (p=0.003). By age- and sex-adjusted logistic regression analysis, frailty was found to be strongly and independently associated with the presence of TCFA (odds ratio [OR] 2.81, 95% confidence interval [CI]:1.06-7.48, p=0.039).
Conclusions: This is the first study to report the relationship between frailty phenotype and coronary plaque morphology among frail older NSTEACS patients. ClinicalTrials.gov Identifier: NCT01933581
Introduction
Our population is rapidly ageing. Twenty-five to 50% of patients with cardiovascular disease can be identified as frail1,2. The prognostic impact of frailty following non-ST-elevation acute coronary syndrome (NSTEACS) has been demonstrated in many previous studies3,4,5,6,7,8,9. However, this high-risk older patient cohort is underrepresented in clinical trials10. Recent trials evaluating an early invasive strategy in the elderly with NSTEACS have lacked statistical power to detect any mortality benefit; frailty was not assessed nor was high-risk plaque phenotype by intravascular imaging11,12,13. Thus, older, frail patients are less likely to receive advanced care including invasive angiography and guideline-directed medical treatment due to uncertainty concerning the risks and benefits14,15.
The main cause of heart disease mortality is rupture of a thin-cap fibroatheroma (TCFA), also known as vulnerable plaque. Coronary artery plaque burden and morphology among older patients and their association with frailty have not been studied. Previous studies investigating plaque composition (using intravascular ultrasound [IVUS]) and adverse cardiovascular outcomes all evaluated a much younger patient population16,17,18. Therefore, the aim of this study was to evaluate the association of frailty with coronary plaque phenotype among older patients with NSTEACS. Whether frailty was independently associated with high-risk plaques was also evaluated.
Methods
STUDY DESIGN
The current study is a subgroup invasive imaging (virtual histology intravascular ultrasound [VH-IVUS] study) analysis of the study to Improve Cardiovascular Outcomes in high-risk patieNts with acute coronary syndrome (ICON1). The ICON1 study was carried out in accordance with the Declaration of Helsinki.
Ethical approval was gained from the National Research Ethics Service (12/NE/01600). Written, informed consent was obtained from all participants prior to enrolment into the study. ICON1 was registered with the United Kingdom Clinical Research Network (UKCRN; ID 12742) and ClinicalTrials.gov (NCT01933581). The ICON1 study protocol has been published previously19.
The ICON1 study was designed as a multicentre prospective observational study of patients aged ≥75 years undergoing invasive management for NSTEACS. Patients referred to two tertiary cardiac centres were recruited between November 2012 and December 2015. One-year follow-up was completed in December 2016. The screening log data from this study have been published previously20. In total, 298 patients were enrolled in the ICON1 study and the one-year clinical outcomes for the whole study have been published21. The current invasive imaging subgroup analysis included data from 90 patients.
The primary outcome measure for this VH-IVUS subgroup study is the prevalence of vulnerable plaque virtual histology thin-cap fibroatheroma (VH-TCFA) in frail and non-frail patients. We hypothesise that frail patients have a greater presence of high-risk lesions including VH-TCFA and that frailty is independently associated with high-risk plaques.
FRAILTY ASSESSMENT
Frailty status was assessed using the Fried frailty criteria derived from the Cardiovascular Health Study, which consists of subjective and objective assessment in five domains: weight loss, exhaustion, physical inactivity, weakness, and slow walking/getting up from chair2. Each criterion provides a score of one point, and the sum is used to define frailty status, a score of 0 being robust, a score of 1 or 2 being pre-frail, and a score ≥3 being frail.
VIRTUAL HISTOLOGY INTRAVASCULAR ULTRASOUND (VH-IVUS) STUDY
Following diagnostic coronary angiography, patients underwent VH-IVUS imaging of all three coronary arteries prior to percutaneous coronary intervention (PCI), where this was feasible and not contraindicated. A 20 MHz, phased array Eagle Eye® Platinum catheter was mounted on an R-100 pullback device and connected to either an integrated s5i system or a mobile s5 tower (all Philips Volcano, San Diego, CA, USA). Image acquisition was performed at a pullback speed of 0.5 mm/s and was ECG-gated. The maximum feasible length of all three coronary arteries was imaged. The data were anonymised and transferred to DVD for off-line data analysis. The operator was blinded to these data.
ANALYSIS OF IVUS DATA
VH-IVUS data analysis was performed in the Newcastle Angiography/IVUS/optical coherence tomography (OCT) core laboratory using the Medis QIVUS software, versions 2.2 and 3.0 (Medis medical imaging systems, Leiden, the Netherlands). Contours were drawn manually around the external elastic membrane (EEM) and lumen of the vessel for each greyscale IVUS frame, excluding any ring-down artefact or previously stented segments. Quantitative IVUS measurements included cross-sectional areas of EEM, lumen, plaque and media area (cross-sectional area of the EEM minus that of the lumen), plaque burden (PB: plaque and media cross-sectional area divided by EEM cross-sectional area), minimum lumen area (MLA) and diameter, percent stenosis, and absolute volume and percentage of total plaque volume reported for each plaque component (fibrous tissue [FT], fibro-fatty tissue [FF], necrotic core [NC], dense calcium [DC]). Percent atheroma volume was calculated as the proportion of the entire vessel wall occupied by atherosclerotic plaque throughout the segment of interest:
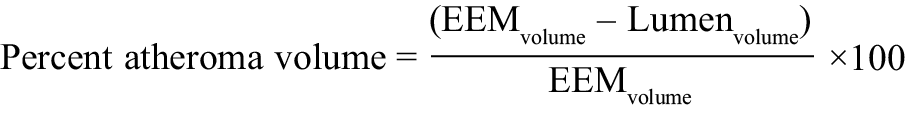
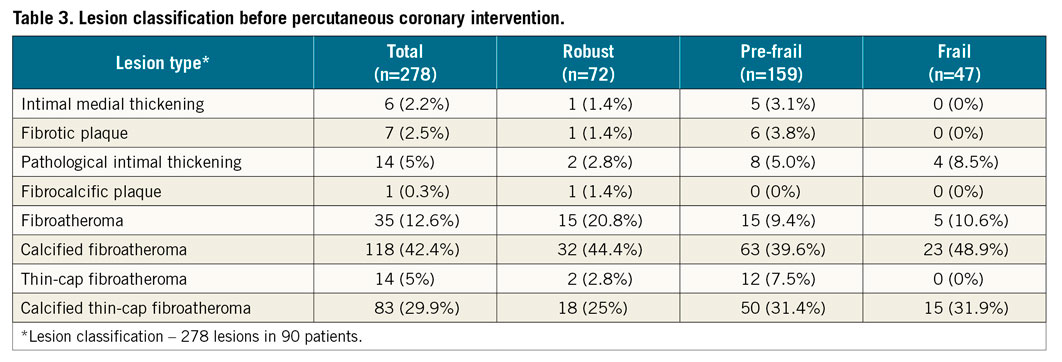
Analyses were performed on VH-IVUS data to determine the lesion type (a lesion was defined as having more than three consecutive slices with ≥40% plaque burden) and a frame-by-frame analysis was then performed to classify the lesion subtype (Supplementary Figure 1) according to definitions from a consensus document22. Percentage plaque phenotype was calculated as: the (number of frames containing plaque phenotype / total number of frames of the vessel) x 100. Lesion-specific data and whole-patient data are presented separately. When a patient had multiple lesions, mean values were taken for calculation of % lesion types.
The composite major adverse cardiovascular events (MACE) was defined as: all-cause death, myocardial infarction (MI), stroke, unplanned repeat revascularisation, bleeding, and all-cause re-hospitalisation at one year. Only the event occurring first was counted. Significant bleeding was defined as per the Bleeding Academic Research Consortium (BARC) criteria23. All one-year outcomes were ascertained at the follow-up appointment.
STATISTICAL ANALYSIS
Assuming that (a) one third of participants would be classified as robust, (b) based on prior studies17,22 high-risk lesions (e.g., TCFA) would be present in 30% of the robust patients, and (c) the combined pre-frail and frail phenotypes would be associated with an approximate relative risk (RR) of 2, a sample size of at least n=93 was required to achieve a power of 80%, with a type I error (α) of 0.0524. At the time of the design of the study, we had planned to recruit 100 of the total 300 patients in the ICON1 study into the invasive imaging study, given the fact that we had anticipated that it would not be feasible to perform three-vessel imaging in all recruited patients.
Continuous variables are presented in the form of mean (standard deviation [SD]) or median (interquartile range [IQR]); discrete variables are presented as absolute numbers (percentage). Tests of normality were performed for all variables. The Student’s t-test or one-way ANOVA was performed for the comparison of normally distributed, continuous variables; the Mann-Whitney U test was used for non-normally distributed variables for independent samples. The chi-square test (χ2) or Fisher’s exact test was used for comparison of discrete variables. The association of relevant risk factors (age, sex, history of hypertension, diabetes, and frailty) with plaque phenotype was examined by logistic regression models in unadjusted models. Age- and sex-adjusted models were also used to examine the relationship between frailty and plaque phenotype.
This substudy was not powered to evaluate clinical outcomes and was thus performed as an exploratory analysis only. The Kaplan-Meier (KM) method was used to estimate composite endpoint-free survival curves; the log-rank test was used to ascertain equality of event-free survival curves among groups. The proportional hazard assumption was tested. A Cox regression model was used to obtain the hazard ratio (HR). A two-tailed p-value <0.05 was used as the threshold for statistical significance. SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA) software was used for all statistical analyses.
Results
STUDY RECRUITMENT AND BASELINE CHARACTERISTICS
Ninety patients (age 80.9±3.8 years, 59 [65.6%] male) were included in the VH-IVUS substudy (Figure 1). The reasons for exclusion are shown in Supplementary Figure 2.
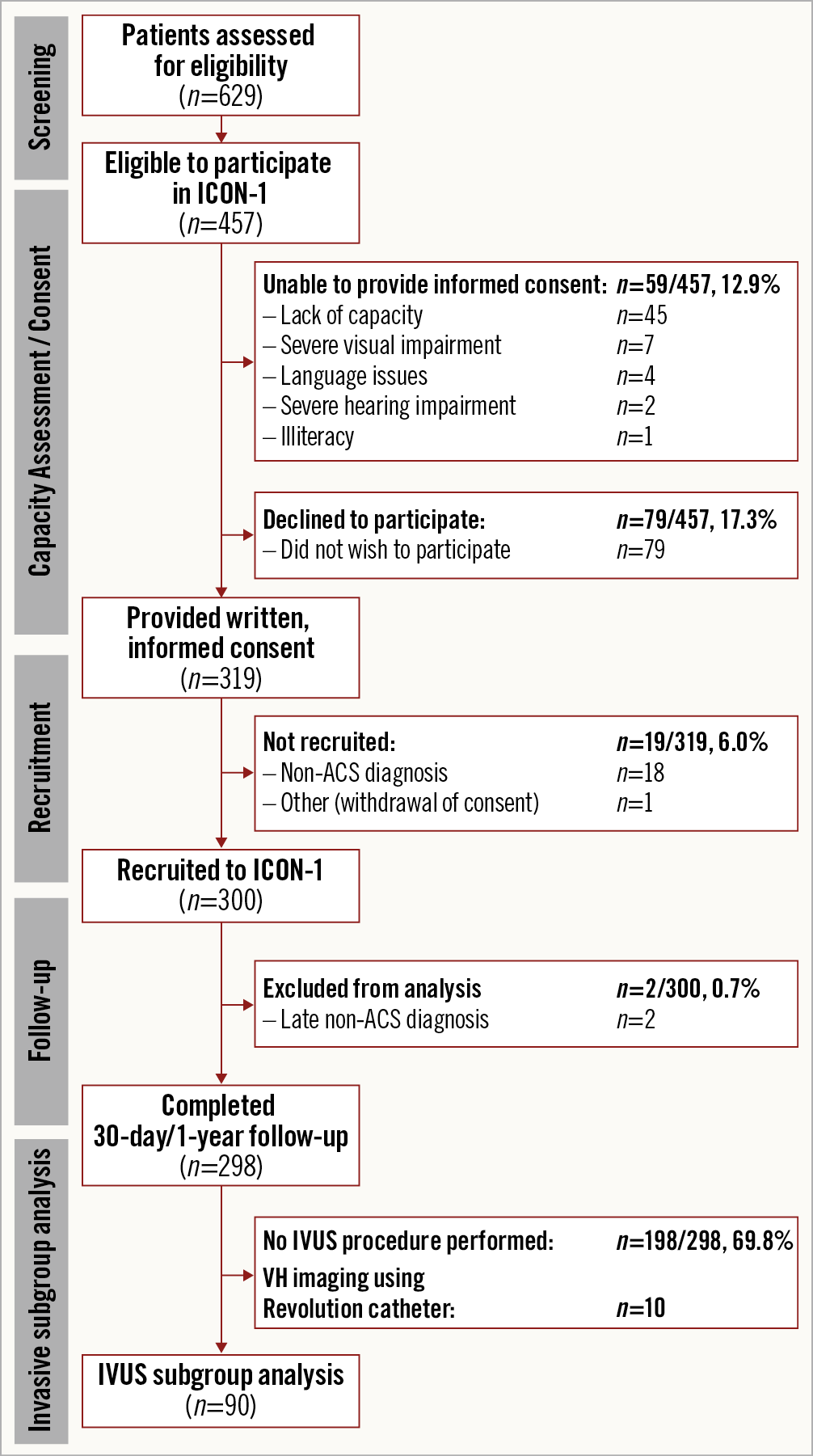
Figure 1. Flow diagram of ICON1 screening, recruitment, and invasive subgroup analysis. ACS: acute coronary syndrome; IVUS: intravascular ultrasound; VH: virtual histology
BASELINE CHARACTERISTICS OF VH-IVUS SUBGROUPS
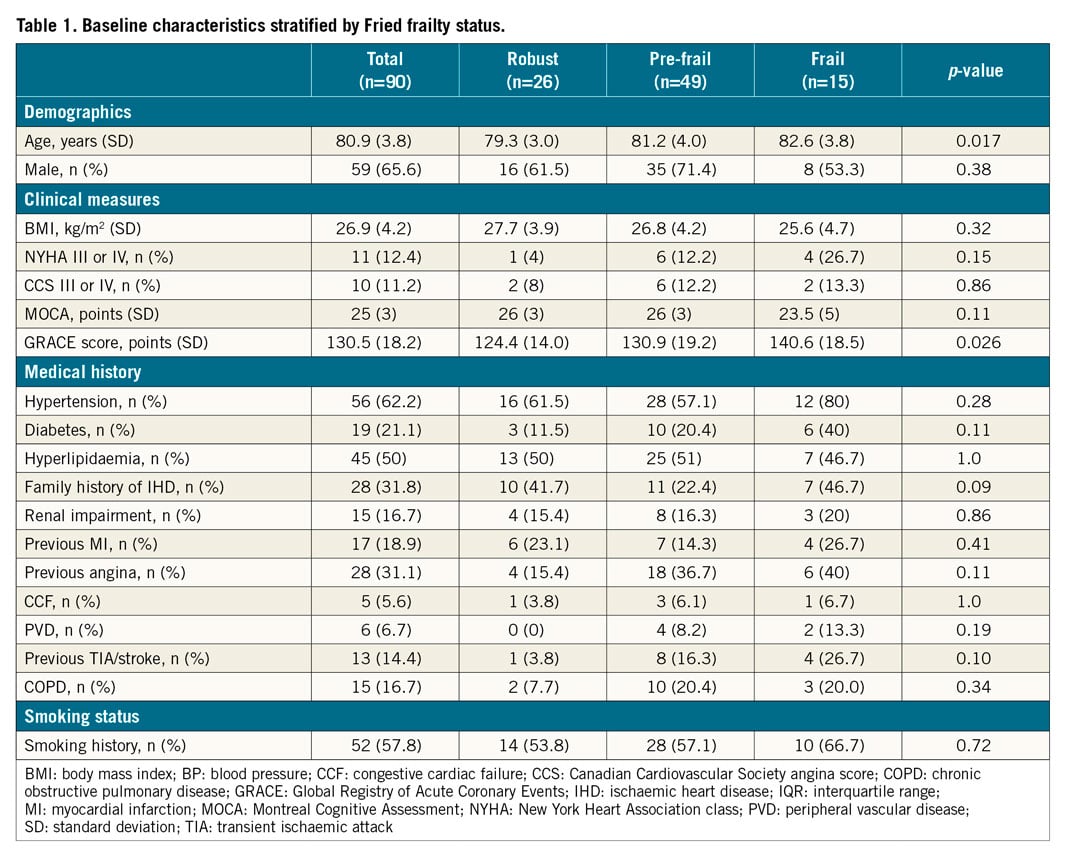
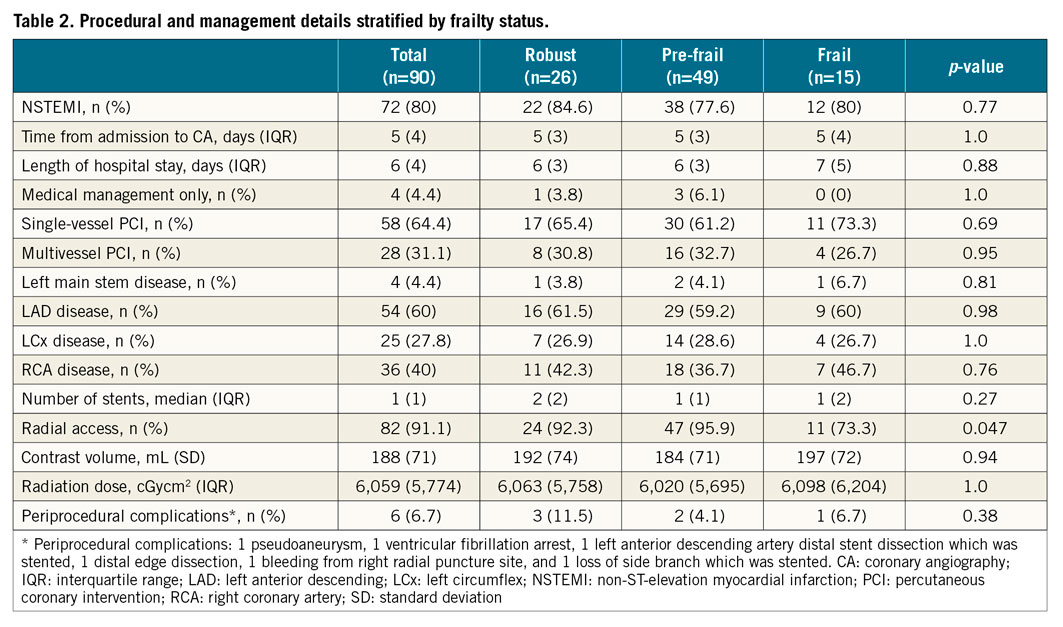
The baseline and procedural characteristics of the study patients are displayed in Table 1 and Table 2. Twenty-six (28.9%) participants were robust, 49 (54.4%) were pre-frail, and 15 (16.7%) were frail. Frail patients were older (p=0.017), with higher GRACE 2.0 scores (p=0.026). The VH-IVUS data are displayed in Table 3, Table 4, Supplementary Table 1 and Supplementary Table 2. The total length of the coronary artery imaged per patient was 97.7 (IQR 82.3) mm, and the median number of IVUS frames analysed per patient was 212 (IQR 182) frames. The pre-frail group was associated with the smallest MLA in culprit lesions (p=0.019, robust vs. pre-frail; p=0.001, pre-frail vs. frail).
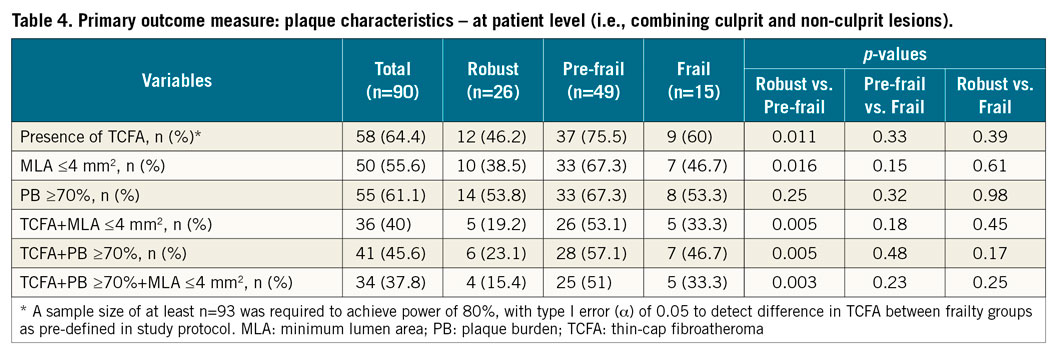
PRIMARY OUTCOME MEASURE
Overall, combining culprit and non-culprit lesions, VH-TCFA (defined as thin-cap fibroatheroma and calcified thin-cap fibroatheroma combined) was present in 58 (64.4%) patients. There was a significant difference in the occurrence of VH-TCFA between robust and pre-frail patients (46.2% in robust vs. 75.5% in pre-frail vs. 60% in frail, p=0.011 for robust vs. pre-frail) (Table 4). Importantly, frailty phenotype (defined as Fried frail and pre-frail combined) was independently associated with the presence of high-risk plaque phenotypes in age- and sex-adjusted logistic regression analyses (Supplementary Table 3): TCFA (odds ratio [OR] 2.81, 95% confidence interval [CI]: 1.06-7.48; p=0.039).
SECONDARY ANALYSIS
Frail patients had the highest DC % to total plaque volume (p=0.02, pre-frail vs. frail; p=0.037, robust vs. frail), and the lowest NC/DC ratio (p=0.004, pre-frail vs. frail; p=0.014, robust vs. frail). In addition to TCFA, patients in the pre-frail group also had the highest proportion of other PROSPECT-defined high-risk plaque lesions: MLA ≤4 mm2 (38.5% vs. 67.3% vs. 46.7%, p=0.016 for robust vs. pre-frail), TCFA+MLA ≤4 mm2 (19.2% vs. 53.1% vs. 33.3%, p=0.005 for robust vs. pre-frail), TCFA+PB ≥70% (23.1% vs. 57.1% vs. 46.7%, p=0.005 for robust vs. pre-frail), TCFA+PB ≥70%+MLA ≤4 mm2 (15.4% vs. 51% vs. 33.3%, p=0.003 for robust vs. pre-frail), with significant difference being found only between the robust and pre-frail groups.
Further analysis was undertaken by analysing the combined pre-frail and frail group vs. the robust group (frail vs. non-frail). The combined pre-frail and frail group had a significantly higher prevalence of PB ≥70% (p=0.03), and % atheroma volume (p=0.02) in the non-culprit artery. The frail group (pre-frail+frail) also had a greater presence of high-risk lesions: TCFA (p=0.02), MLA ≤4 mm2 (p=0.04), TCFA+MLA ≤4 mm2 (p=0.01), TCFA+PB ≥70% (p=0.006), and TCFA+PB ≥70%+MLA ≤4 mm2 (p=0.005).
Importantly, frailty phenotype (defined as Fried frail and pre-frail combined) was also independently associated with the presence of other high-risk plaque phenotypes on logistic regression analyses (Supplementary Table 3): MLA ≤4 mm2 (OR 3.07, 95% CI: 1.15-8.2), the presence of TCFA+MLA ≤4 mm2 (OR 4.07, 95% CI: 1.34-12.42), TCFA+PB ≥70% (OR 3.79, 95% CI: 1.32-10.91), and the presence of all three combined (TCFA+PB ≥70%+MLA ≤4 mm2; OR 4.89, 95% CI: 1.48-16.13).
EXPLORATORY CLINICAL OUTCOMES DATA
Eighty-nine patients completed one-year follow up. Frail patients had a higher MACE rate (p=0.04) driven by a higher rate of all-cause rehospitalisation (p=0.013) (Supplementary Table 4). Twenty-eight rehospitalisation episodes occurred in 25 patients, comprising admissions due to: cardiovascular (10; 35.7%), gastroenterological (5; 17.9%), neoplastic (3; 10.7%), renal (2; 7.1%), pulmonary (1; 3.6%), neurological (1; 3.6%), and other (6; 21.4%) causes.
At one year, the pre-frail/frail phenotype was associated with higher MACE (p=0.04) by KM survival analysis (Supplementary Figure 3A, Supplementary Figure 3B). Although there was an indication of violation of the proportionality assumption from KM curves, the Cox regression model provided a reasonably well-fitted survival curve to the observed survival curve (Supplementary Figure 4) and was therefore used to identify predictors of a MACE endpoint. Relative to frail patients, robust patients had less chance (HR 0.37, p=0.03) of reaching MACE. Additional analysis was also performed, demonstrating a non-significant difference in survival curves in individual and combined plaque phenotypes (Supplementary Figure 5).
Discussion
The present study demonstrated for the first time that, in older patients undergoing invasive management of NSTEACS, high-risk coronary artery lesion phenotypes were more common in frail and pre-frail compared to robust older patients. Frailty is strongly and independently associated with PROSPECT-defined high-risk plaque phenotypes.
Several previous studies have examined coronary artery plaque phenotypes by VH-IVUS and their relationship with clinical outcome, including the Providing Regional Observations to Study Predictors of Events in the Coronary tree (PROSPECT) study18, the Virtual Histology in Vulnerable Atherosclerosis (VIVA) study16, and the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis - Intravascular Ultrasound (ATHEROREMO-IVUS) study17. These previous three main VH-IVUS clinical outcomes registries evaluated a much younger patient population (PROSPECT: median age 58.1 years; VIVA: median age 63.1 years; ATHEROREMO-IVUS: mean age 61.6 years); frailty phenotype was not examined. Our study is the first report of an association between VH-IVUS-based plaque classification and patient frailty phenotype in this growing high-risk older patient population. Overall, the presence of vulnerable plaque was shown to be high (64.4% at patient level in ICON1; 50.2% in PROSPECT; and 41.7% in ATHEROREMO-IVUS).
A number of important differences between our study (ICON1) and previously reported studies may explain the higher prevalence of high-risk coronary lesion phenotypes. Firstly, the ICON1 study included a much older, high-risk population which may be associated with a higher prevalence of high-risk plaques. Secondly, our study contained high-risk NSTEACS patients, whereas VIVA and ATHEROREMO-IVUS contained both stable angina and ACS patients. Thirdly, the VH-TCFA definitions varied among studies. The VIVA and ATHEROREMO-IVUS studies both required confluent necrotic core >10% of plaque cross-sectional area to be in contact with the vessel lumen for three consecutive frames; the PROSPECT study required confluent necrotic core of >10% plaque area to be in contact with the lumen for a ≥30º arc for three consecutive frames. In ICON1, we used the definition set by the published consensus document which updated and unified VH-IVUS imaging analysis and plaque definitions after the PROSPECT, VIVA, and ATHEROREMO-IVUS studies were conducted, where confluent necrotic core >10% of plaque area was required to be in contact with the lumen for a >36º arc for three consecutive frames. This may have reduced the proportion of lesions being reported by VH-IVUS; thus, the “actual” VH-TCFA prevalence may be even higher in this high-risk older patient group. Fourthly, we obtained IVUS images prior to PCI including both culprit and non-culprit lesions, whereas only non-culprit lesions were analysed in PROSPECT as IVUS images were obtained after PCI. Furthermore, the In-Vision Gold console (Philips Volcano) was used in PROSPECT instead of the s5 for the VH algorithm used in our study; the amount of necrotic core detected may therefore differ.
No previous study has shown the association of frailty with coronary artery plaque phenotype. For the first time, in our study we have shown that frailty was independently associated with high-risk plaque phenotype which is a novel finding that might explain the association of frail patients with adverse outcomes. Importantly in our study, radial access was achieved in 91.1% of cases. The Fried frailty assessment (slow walking/getting up from chair component) would not have been affected in the majority of patients. Frail older patients are often denied advanced care, including angiography and revascularisation, due to fear of futility and complications. The definitive benefit of coronary angiography and revascularisation among frail older patients (≥75 years of age) presenting with NSTEMI is currently being evaluated in the ongoing British Heart Foundation SENIOR-RITA trial (ClinicalTrials.gov NCT03052036).
Study limitations
The ICON1 study recruited patients who have been referred to tertiary cardiac centres for coronary angiography, and it is thus possible that the oldest and frailest patients who were not offered invasive management were not included in our study. This current subgroup analysis study was also limited by the selective patient cohort, with particular coronary anatomy features suitable for VH-IVUS imaging and PCI, constraints from cardiac catheter laboratory operators, other urgent cases waiting, and time and other constraints in the catheter laboratory (Supplementary Figure 2). Furthermore, the small sample size also restrains statistical power to determine association of plaque phenotype with clinical outcomes. Nevertheless, for the first time our study has provided important insights into the coronary artery plaque phenotype in this older frail patient cohort. Moreover, we attempted to recruit very high-risk frail older patients involving a complex invasive study protocol. The study was successfully executed with important unique findings in this patient cohort.
Conclusions
This is the first study to demonstrate differences in the coronary plaque phenotype among frail older patients presenting with NSTEACS. Frailty is strongly and independently associated with high-risk plaque phenotypes including TCFA.
|
Impact on daily practice Frail, older patients are at higher risk of poor outcomes following an acute coronary syndrome. In our study, frailty was strongly and independently associated with high-risk plaque phenotypes, which might contribute to adverse events in this group of patients. Older patients should be offered contemporary treatments to improve their clinical outcomes. |
Acknowledgements
Carmen Martin-Ruiz and Gabriele Saretzki, Institute for Ageing and Health, Newcastle University, for biomarker analysis support. Newcastle angiographic/IVUS/OCT core laboratory members: Vijay Kunadian, Hannah Sinclair, Kimberley Batanghari, Dhiluni Kandage, Jin Tee, Ross Fowkes, Victor Tsoi, Benjamin Beska, Rebecca Jordan, Amy Burrell, Shristy Subba, James Latimer. The cardiology research team at Freeman Hospital: Kathryn Proctor and Jennifer Adams-Hall for participant follow-up support. Professor Javed Ahmed, Professor Rajiv Das, Dr Alan Bagnall, Professor Ioakim Spyridopoulos, Professor Azfar Zaman, Dr Richard Edwards, Dr Mohaned Egred, Dr Ian Purcell, Freeman Hospital, Newcastle upon Tyne, UK; Dr Mark de Belder and Bev Atkinson, James Cook University Hospital, Middlesbrough, UK, for data collection support.
Funding
The research was supported/funded by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre (BRC) based at Newcastle Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. This study was also supported by unrestricted research support from Volcano Corporation, San Diego, USA. V. Kunadian has received research funding from NIHR BRC Newcastle, AstraZeneca (ISSBRIL0303), and the British Heart Foundation (CS/15/7/31679).
Conflict of interest statement
The authors have no conflicts of interest to declare.

