Abstract
Aims: A bipolar multi-electrode 7 Fr-compatible balloon-catheter radiofrequency (RF) renal denervation system (Vessix™ Renal Denervation System; Boston Scientific, Marlborough, MA, USA) was evaluated for safety in domestic swine.
Methods and results: Renal arteries of 27 swine received overlapping treatments proximally/single treatments distally to mimic balloon overlap clinically. Each histopathology cohort (30, 90, 180 days) had four RF-treated and three sham-treated (no RF energy delivered) animals, with the response of artery/surrounding nerves to bilateral treatment examined (42 arteries). Scanning electron microscopy of the renal artery flow surface for endothelialisation was performed in six additional pigs (three at each of 30 and 90 days: 12 arteries) following unilateral whole artery treatment with proximal overlap: RF one side, sham the other side. Power was ~1 watt, treatment duration 30 seconds, target temperature 68°C. Renal histology and assessment for off-target injury was performed in all 27 swine. Renal artery thermal injury was transmural and segmental involving <10% to >90% of the circumference (typically 30-60%) with segmental neointimal hyperplasia exceeding shams but haemodynamically trivial (maximum stenosis 17.7%). Healing of necrotic arterial media was by replacement fibrosis. Overlying nerves also became fibrotic. Endothelialisation was focally incomplete at 30 days but confluent at 90 days. No off-target injury occurred outside the renal arteries.
Conclusions: Safety was demonstrated.
Introduction
Catheter-based renal sympathetic denervation is a potential treatment for drug-resistant systemic hypertension, and multiple endovascular renal denervation systems are under development. In this study, the safety of one such system, the Vessix™ Renal Denervation System (Boston Scientific Corporation, Marlborough, MA, USA), was evaluated in normotensive healthy common swine.
The Vessix system is designed to deliver radiofrequency (RF) energy to damage thermally, and thereby inactivate, sympathetic nerves located in the renal artery adventitia. The system’s balloon catheter and bipolar electrodes are designed to create limited, precisely located lesions, avoid any thermal injury to off-target tissues such as ureters, kidneys, adrenal glands and bowel, and yet ablate most of the sympathetic nerves supplying the kidneys.
In this preclinical safety study, full artery-length treatment was typically achieved with one or two adjacent treatments. However, to achieve a robust, conservative safety assessment, an additional balloon inflation was performed in the proximal renal artery to simulate unintended RF treatment overlap.
Methods
EXPERIMENTAL DESIGN
The Vessix Reduce™ Catheter was applied bilaterally to 54 renal arteries in 27 pigs divided into three cohorts (Table 1), not counting one animal which died intraoperatively before treatment and was replaced. The response of the renal arteries and surrounding nerves was examined histopathologically, and scanning electron microscopy (SEM) was used to assess the renal artery flow surface.
All animals in both the histopathology and the SEM cohorts underwent a necropsy examination which included a detailed search for off-target lesions from thermal energy. All 54 kidneys, as well as other tissues showing gross observations suspicious for thermal injury or any other pathology, were sampled for histology.
DEVICE DESCRIPTION AND RENAL DENERVATION PROCEDURES
The Vessix system used in this study comprises the Vessix generator and the Vessix Reduce Catheter, an over-the-wire low-pressure (three atmospheres) 7 Fr-compatible balloon catheter. The 25 mm long non-compliant polyethylene terephthalate balloon has surface-mounted gold-containing bipolar electrodes positioned to achieve a helical treatment along the artery, as illustrated in Figure 1. Balloons with diameters of 5, 6, or 7 mm (chosen to avoid oversizing renal arteries by more than approximately 30%) and four to six bipolar electrodes, depending on balloon size, were used in this study.
![]()
Figure 1. Illustration of the Vessix balloon catheter, showing helical placement of the bipolar electrodes. Used with permission of Boston Scientific Corporation. ©2014 Boston Scientific Corporation or its affiliates. All rights reserved.
The RF generator provides visual prompts to guide the operator through the set-up and treatment steps. The system measures electrical impedance to determine electrode apposition to the renal artery wall and the generator delivers low-power (approximately 1 watt) radiofrequency energy simultaneously to all apposed electrodes according to a pre-programmed temperature profile. Thermistors on the balloon surface monitor tissue temperature throughout the 30-second treatment period and the generator adjusts energy delivery to achieve a target temperature of 68°C.
Following induction of anaesthesia in each pig, the 90 cm long Vessix Reduce 7 Fr-compatible percutaneous balloon catheter was introduced into the abdominal aorta through a femoral artery and the uninflated balloon positioned in a renal artery. The balloon was initially positioned distally in the renal artery with the balloon tip advanced to the arterial bifurcation, inflated to achieve electrode apposition to the renal arterial wall and, for RF-treated arteries, a single RF treatment given. Following this single distal RF treatment, the balloon was deflated and pulled back to treat any untreated proximal portion of the renal artery. Following the proximal treatment, the balloon was again deflated and repositioned to perform a second (overlap) treatment in the proximal 12 mm of the renal artery. Sham-treated animals underwent the same pauses as RF treatment (i.e., 30 seconds) but no energy was applied.
CLINICAL PATHOLOGY: HAEMATOLOGY AND CHEMISTRY
Standard haematology and serum chemistry profiles together with urinalysis were obtained at baseline (day 0 just prior to intervention), day 7(±1 day) and, depending on the cohort, at day 45±5, day 90±5 and just prior to termination at 30, 90 or 180 days.
ANATOMICAL PATHOLOGY: NECROPSY, HISTOLOGY AND SCANNING ELECTRON MICROSCOPY
NECROPSY EXAMINATION
At necropsy, gross examination of any pathological finding was conducted with particular emphasis on evidence suggestive of non-target injury to the kidneys, adrenal glands, ureters, renal veins, psoas muscles, peritoneum and intestines. Any grossly abnormal tissues were histologically examined.
HISTOLOGICAL PROCESSING AND HISTOPATHOLOGICAL ASSESSMENT
The renal artery/renal veins, associated abdominal aorta, and the kidneys, including the proximal ureters, were removed and pressure-fixed overnight at 75-100 mmHg with buffered 10% formalin. As much adventitial fibro-fatty tissue as possible was retained with the renal arteries. Following pressure fixation, the renal arteries together with adherent adventitia, including nerves, were removed, further immersion fixed for over 24 hours and then divided into transverse tissue samples at approximately 3 mm intervals for conventional histology. Two histological sections were prepared from each tissue block, one stained with elastic trichrome and the other with haematoxylin and eosin.
Renal artery histopathological analysis included every histological section, but a proximal, middle, and a distal section were chosen from each vessel for more detailed morphological and morphometric evaluation subject to statistical analysis. Maximum neointima production was prioritised as a criterion for section choice.
Seven morphologic parameters were assessed: endothelial cell coverage, luminal thrombus, loss of internal elastic lamina (IEL), loss of external elastic lamina (EEL), medial smooth muscle cell (SMC) loss, medial inflammation, and adventitial inflammation. Seven morphometric parameters were evaluated: lumen area, lumen equivalent diameter, IEL area, neointimal area (IEL area - lumen area), EEL area, medial area (EEL area - IEL area) and IEL-based percentage area stenosis ([1–lumen area/IEL area]×100%). Also, the percentage of the circumference occupied by fibrosis, if transmural, was estimated.
Nine haematoxylin and eosin stained sections from each kidney (three each from the upper, middle and lower thirds of the organ) were examined.
SCANNING ELECTRON MICROSCOPY
The entire renal arterial flow surface was observed en face with a Hitachi TM3000 environmental microscope (Hitachi, Tokyo, Japan) and endothelial cell coverage, luminal thrombus and the presence of inflammatory cells (leukocyte adherence) were graded (Online Table 1) at 100 dwell points distributed uniformly over the flow surface in a 10×10 array (i.e., the vessel circumference and length from renal artery ostium to renal artery bifurcation each divided into 10 equal parts). The flow surface of aorta and the renal artery beyond the bifurcation were also examined.
Statistical analysis
Morphometric and morphologic data were compared between the RF-treated and sham-treated groups at each follow-up time point (30, 90, 180 days), across time points within each treatment cohort, and between overlap treatment and single treatment at each time point. For comparisons between RF-treatment and sham, and across time points, the worst-case values for each morphometric and morphologic parameter were used from one of the three selected (proximal, middle, distal) histological sections for each renal artery. Worst-case morphometric data for each artery consisted of the minimum lumen area, lumen equivalent diameter, IEL area and medial area, and the maximum value of EEL area, neointimal area and percent area stenosis. Worst-case morphologic scores consisted of the highest digit in the ordinal scores (Online Table 1) for morphologic grading scales. For comparison of single and overlap treatment, the morphometric and morphologic parameters from the proximal sections (overlap treatment) were compared with those from the distal sections (single treatment) for RF- and sham-treated groups.
Morphometric data were compared using a general linear model (GLM) to compare means within each time point and across time points. If GLM results showed statistically significant differences, the Tukey pairwise comparison method was applied to determine if differences in means were statistically significant between treatment groups (RF versus sham) at each time point and between time points for each treatment group. Paired t-tests compared overlap to single treatment data. Morphologic data were compared using the Kruskal-Wallis nonparametric method and the Mann-Whitney rank-sum test to determine statistically significant differences in medians. Paired t-tests compared overlap to single treatment data. Statistical significance was designated as p≤0.05 for all statistical testing methods.
Results
IN-LIFE OBSERVATIONS AND UNSCHEDULED DEATHS
No in-life observations were considered clinically significant, including all blood and urine testing.
Two of the original 28 swine in the study had early mortality which was unrelated to RF treatment. One animal died intra-operatively before treatment and was replaced. Another animal in the 180-day cohort died on day 131 with torsion of the spiral colon and stomach perforation, the latter attributed to post-mortem gas build-up observed at necropsy. Gross examination combined with histopathology supported 180 degree torsion of the spiral colon as the cause of death. This animal was in the RF-treatment group, and, significantly, there were no RF treatment-associated injuries to off-target tissues. Renal artery and kidney histopathology were similar to the other RF-treated animals in this cohort.
OBSERVATIONS AT NECROPSY
Careful examination for gross evidence of pathology in the 26 swine which collectively reached the 30, 90 and 180-day time points yielded no pathology findings except for an occasional incidental renal cyst.
KIDNEY HISTOPATHOLOGY
Renal histology was obtained from every kidney of all 27 swine; no microscopic findings indicated either embolic or thermal injury. Small chronic inflammatory foci consisting predominantly of lymphocytes, sometimes with macrophages also present, were occasionally seen in both RF-treated and sham-treated animals, with essentially the same appearances in the 30, 90, and 180-day cohorts. No foreign material was identified in these minor inflammatory foci. This inflammatory activity is of no practical consequence.
RENAL ARTERY HISTOPATHOLOGY
In the RF-treated vessels at 30, 90 and 180 days post treatment, foci of segmental transmural replacement fibrosis (indicating previous thermal injury to the media) included variable percentages of the circumference, typically 30-60%, but in a few instances up to 90-100%. Either one or two zones of segmental transmural replacement fibrosis were common. Both patterns are shown in Figure 2. Average transmural medial fibrosis as a percentage of circumference is presented in Table 2. Thermal injury healing was by replacement fibrosis with the necrotic SMCs replaced by extracellular matrix (predominantly collagen).
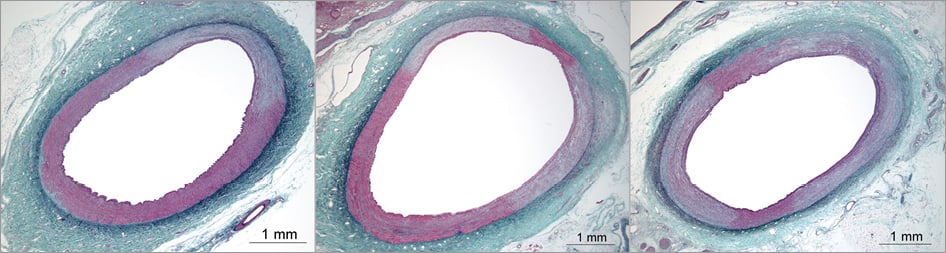
Figure 2. Renal artery thermal injury (RF treatment) was characteristically segmental and transmural, varying from one zone of thermal injury (typically ~25% of the circumference, left) through two zones (typically ~40% of the circumference, right). Elastic-trichrome stain.
By 30 days post RF treatment, inflammatory activity was typically minimal to mild in the media and mild in the adventitia. At both 90 and 180 days, inflammation was generally minimal in both media and adventitia, never exceeding mild in any section. The healing sequence is shown in Figure 3. At 30 days, replacement fibrosis was well advanced but “islands” of necrotic SMCs still remained. At 90 days, healing was essentially complete with mature replacement fibrosis which looked similar at 180 days.
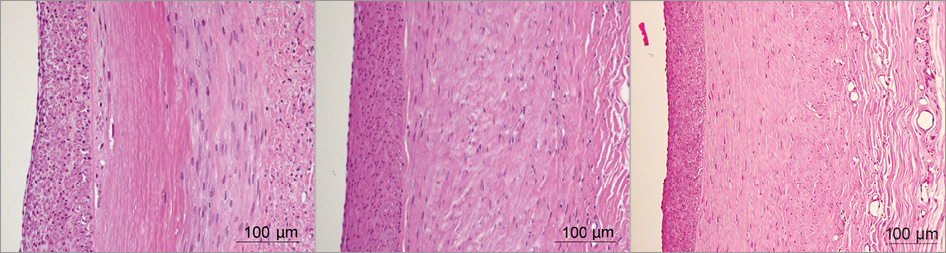
Figure 3. The healing sequence following thermal (RF treatment) injury progressed from transmural medial necrosis with early replacement in which islands of necrotic smooth muscle cells remained in the media, at 30 days (left), to complete replacement fibrosis of the media at 90 days (middle) with a less cellular media with more mature fibrosis at 180 days (right). There was neointima formation at 30 days and this progressed to become more mature (less cellular) through 90 and 180 days. Haematoxylin and eosin stain.
As expected, no segmental transmural thermal injury with developing replacement fibrosis was seen in the sham-treated vessels. There was, however, evidence of focal mechanical injury with characteristic focal loss of IEL and developing localised fibrosis, which was usually shallow but sometimes deep. Unlike with thermal injury, fibrosis was not in a segmental, transmural pattern. Of note, IEL was usually well preserved over wide swathes of transmural thermal injury. Occasional combinations of responses to both thermal and mechanical injury were seen in RF-treated vessels, as expected.
RF treatment sufficient to cause initial segmental transmural medial necrosis (necessary for the nerves outside the renal artery to be thermally injured) most certainly causes concurrent necrosis of endothelial cells lining the renal artery flow surface. Balloon inflation alone may be sufficient to damage the endothelium. However, endothelium regenerates from the viable reservoir around the thermal lesions and any mechanical injury caused by the balloon. At 30, 90 and 180 days, this endothelial regeneration was complete on histological evaluation. No luminal thrombus whatsoever was seen in any renal artery sections. It is likely that there was some luminal thrombus acutely, which became organised into developing, endothelial cell-covered neointima.
Focal neointimal hyperplasia was seen in response to both thermal and mechanical injury to the renal arteries. Neointimal proliferation was minimal or mild, always haemodynamically trivial, and did not meaningfully progress between 90 and 180 days. Typical area percent stenosis was 5-10%. The maximum percent area stenosis in any section was 14.0% for the 30-day cohort, 17.7% for the 90-day cohort and 15.8% for the 180-day cohort. These maximums were each for RF-treated vessels, as expected. Figure 4 shows the maximally stenotic sections at 90 and 180 days with lumen, IEL and EEL tracing to illustrate the modest amount of neointima compared to the lumen size. Of note, five renal artery sections had essentially circumferential (90-100%) transmural fibrosis healing thermal injury, yet none of these had percent area stenosis exceeding 13.5%.
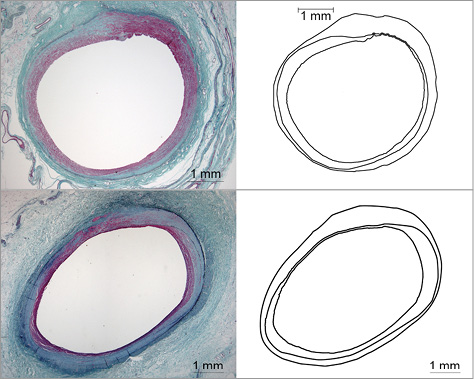
Figure 4. The histological sections showing maximal luminal narrowing (area percent stenosis), together with their outline drawings showing lumen, IEL and EEL, are presented for the 90-day (upper) and 180-day (lower) cohorts; both are from RF treatment. Elastic-trichrome stain.
Nerves adjacent to the segmental zones of transmural arterial thermal injury also showed thermal injury. A loss of capillaries was seen within the nerves overlying the RF-treated renal arteries, together with early fibrosis within and around the nerves at 30 days. At 90 and 180 days, nerves adjacent to the transmural, segmental zones of thermal arterial injury healed by replacement fibrosis also showed marked fibrotic changes with nerve bundles broken up by tongues of invading fibrosis. These features are shown in Figure 5.
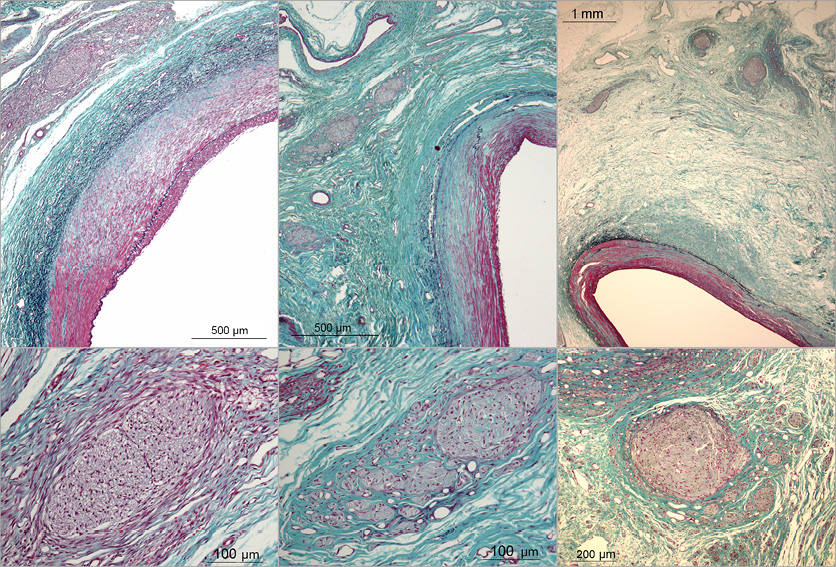
Figure 5. Representative histopathology showing nerve injury, always overlying transmural segmental regions of arterial thermal injury, is presented for 30 days (left), 90 days (middle) and 180 days (right). At 30 days there was early fibrosis both within and around nerve bundles (left). At 90 and 180 days tongues of fibrous tissue split up fibrotic nerve bundles (middle and right). Elastic-trichrome stain.
SCANNING ELECTRON MICROSCOPY
In the 90-day cohort, all 600 observed dwell points (i.e., 100 dwell points each for six vessels) were scored 0/0/0 for endothelial cell coverage, luminal thrombus and inflammatory cells (Online Table 1). The photomicrographs demonstrated complete endothelial cell confluence (no spaces between endothelial cells). At 30 days, no thrombus and very few or no inflammatory cells were seen at any of the 600 dwell points in the six renal arteries examined. However, four of the six arteries had many instances of incomplete endothelial cell coverage graded as either grade 1 or 2.
Figure 6 shows SEM photomicrographs comparing 30 and 90-day observations. Almost all of these grade 1 and grade 2 observations were in the portion of the artery distal to the overlap zone which had been subjected to repeated balloon inflation either with or without heat. Furthermore, these observations of incomplete endothelial cell coverage were seen more often in sham-treated than in RF-treated arteries. These findings suggest that the foci of incomplete flow surface endothelial cell coverage were not specifically related to thermal treatment. In one 30-day animal, both renal arteries had all 0/0/0 scores as seen in the 90-day cohort.
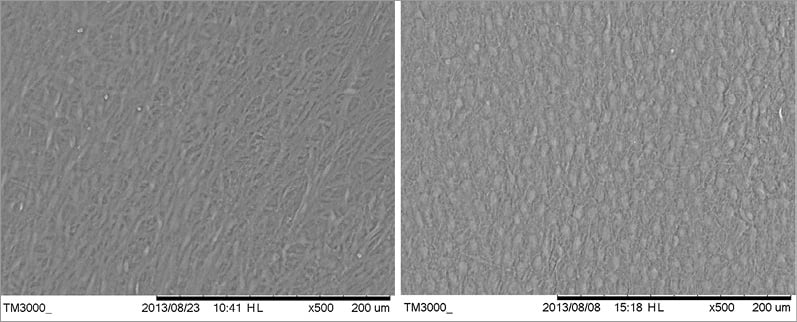
Figure 6. On SEM at 30 days (left), endothelial cell confluence was seen on 81% of the overall flow surface but not completely confluent on the remainder (worst case shown). At 90 days (right), complete endothelial cell confluence was seen in 100% of the flow surface.
The 30-day SEM observations do not raise a safety concern because no associated luminal thrombus was seen on the flow surface in even a single instance. The occurrence of luminal thrombus could raise a safety concern, either related to detachment with thromboembolism or exposure of thrombus to narrow or occlude the renal arterial lumen.
It is conceivable that there might have been some luminal thrombus which embolised earlier than 30 days. If so, the amounts were too small to detect in the histological survey of the kidneys, which included animals designated for SEM.
STATISTICAL ANALYSIS OF RENAL ARTERY HISTOPATHOLOGY
Markedly variable pressure fixation with three to fourfold vessel-to-vessel variability in EEL areas in both sham- and RF-treated groups precluded valid comparisons of lumen area, lumen equivalent diameter, IEL area, EEL area and area percent stenosis. Comparisons for neointimal area and medial area, little affected by pressure fixation, are, however, valid. Neointimal area was significantly greater with RF than with sham treatment at both 90 and 180 days. Scores for medial SMC loss were significantly higher with RF treatment than sham treatment at 30, 90 and 180 days. Medial area and adventitial inflammation scores were greater for RF treatment than sham treatment, but only at 30 days. Greater neointimal formation is expected in response to greater medial injury, and there was much more medial injury with RF treatment than sham treatment.
Scores for loss of IEL were significantly higher for RF-treated vessels in comparison to sham-treated vessels only at 90 days. The same applied to loss of EEL at both 90 and 180 days. These results seem reasonable as RF-treated vessels were subjected to both thermal and mechanical injury and both IEL and EEL loss occur by elastolysis and tend to accumulate over time. However, the RF-treated group did quantitatively seem to have more mechanical injury (which affects IEL more than EEL) than the shams, which may merely have been coincidental.
For comparisons across time points, neointimal area was significantly greater at 90 days than 30 days for the RF-treated vessels but not greater at 180 days than 90 days. This was expected and indicated neointimal formation stabilised before 90 days. Scores for IEL loss were also significantly higher for RF-treated vessels at 90 days compared to 30 days, but not between 90 and 180 days. This suggests that IEL loss, which occurs due to enzymatic elastolysis from inflammatory activity and SMC migration, did progress between 30 and 90 days and stabilised between 90 and 180 days.
Comparing between overlap (proximal) and single (distal) treated vessel segments, neointimal area was significantly greater with overlap RF treatment, but only at 180 days, and luminal narrowing was haemodynamically trivial. There was also significantly greater EEL loss with overlap RF treatment only at 180 days. This is not surprising given the combination of thermal and mechanical injury with overlap RF treatment and the opportunity for accumulating elastic tissue loss over time.
Discussion
Taking into account the gross observations at necropsy, clinical status of the animals, kidney histology and renal artery histology together with the SEM of the renal artery flow surface, the Vessix 7 Fr Renal Denervation System is considered safe as it was operated in single and overlap treatments in this preclinical safety study. The key parameters for controlled RF thermal injury are power, time and tissue temperature. It is important to emphasise that safety evaluation is inherently limited to the specific manner in which the catheter is operated in extrapolation from the animal model to the clinical setting and is further limited by the maximum follow-up duration of 180 days in predicting responses in humans over several years.
In assessing safety, we recognise that there was substantial transmural injury to the renal arterial media, healing to replacement fibrosis averaging 40-60%, but sometimes over 90%, of the circumference. With so much medial fibrosis, the potential for long-term aneurysm formation becomes a consideration. The overall elastin network integrity (which includes many layers of elastica in the adventitia as well as the IEL and EEL) is important for dimensional stability of the vessel, but this integrity was never threatened in any histological section in this study.
In this study, overlap treatment of a portion of renal artery in the clinical setting was simulated. Overlap RF treatment was almost indistinguishable from single RF treatment on evaluation by arterial histology. On statistical analysis, there was a detectable increase in the extent of neointima formation in response to overlap treatment. However, this was a modest difference which was only statistically significant at 180-day follow-up. Renal arterial narrowing (area percent stenosis) was haemodynamically trivial, never exceeding 20% in any renal artery section at any time point. As expected, medial SMC loss was significantly greater in RF-treated than in sham-treated vessels at 30, 90 and 180 days. Neointimal area was significantly greater in RF-treated vessels than sham-treated vessels at both 90 and 180 days, reflecting the greater combined effects of thermal and mechanical injury versus mechanical injury alone.
Bipolar operation is an important safety feature of the Vessix system design. By contrast, in unipolar renal denervation the electrical current flows from electrodes on the catheter through tissues, which may include the kidneys, adrenal glands, ureters, bowel and psoas muscles, to an external ground plate. Energy dissipation in these off-target tissues may cause unintended thermal damage1. Focal coagulative necrosis of psoas muscle, and even a kidney or possibly an adrenal gland, is unlikely to have clinical significance but a bowel injury could be problematic. With bipolar operation the electrical current passes between the local electrode poles and much less power is used than with unipolar systems. Thus, off-target thermal injury is greatly reduced: none was found in a careful search at necropsy in any animal in this study. Theoretically, a bipolar system might be expected to produce more medial injury than a unipolar system. In our experience with both in this porcine model, the arterial injury depth must be transmural to achieve substantial denervation, and the circumferential extent of injury (<10% to >90%) was similar.
Fundamental principles in designing a preclinical renal denervation safety study include: 1) instrumentation of both renal arteries, as would occur in the human for substantial deactivation of the sympathetic innervation of both kidneys; and 2) follow-up durations sufficiently long to observe complete or near complete healing. This was achieved in the present study with little additional change in the histopathology of renal arteries or nerves between 90 and 180 days.
Inflammation is expected in response to thermal injury and is necessary for healing. The practical requirement for safety is that the inflammatory response declines and substantially resolves over time, as occurred in this study. There was no example of more than mild inflammation at 180 days, and almost all renal artery histological sections showed minimal mural and adventitial inflammation at 90 and 180 days.
Limitations
This study was limited to safety evaluation. Treatment efficacy was not assessed. Any potential evaluation of efficacy in normotensive swine can come only from surrogate markers, not changes in blood pressure. Measuring tissue norepinephrine concentrations has been suggested as a proxy for assessing renal denervation efficacy in this animal model. However, accurate renal norepinephrine quantification in swine kidney tissue requires precise sample preparation and sensitive/specific assay methodologies. In our experience, there may be considerable animal-to-animal variability in renal norepinephrine levels under baseline untreated conditions, and renal tissue norepinephrine can only be measured at one time point, at euthanasia. These practical limitations translate into comparing mean levels for a treated animal cohort with untreated (or sham-treated) controls. Given the limited cohort sample sizes of this study, which was powered for safety evaluation only, this methodology is inadequate for efficacy evaluation.
Histological assessment of thermal nerve injury in our study did consistently indicate thermal nerve injury with nerve fibrosis well developed at 90 and 180 days and never seen in the shams. However, we did not evaluate nerve injury and healing using a score-based histological assessment system. Such scoring systems are inherently limited because of their qualitative nature. In our view, on histology a quantitative approach to efficacy is necessary and the methodology for this has not yet been fully developed. Also, we did not perform nerve immunohistochemistry, which offers potential for surrogate efficacy evaluation.
Both renal tissue norepinephrine concentrations and nerve immunohistochemistry methodologies were explored in a study2 reporting on the balloon electrode sizing/location and the development of the temperature control algorithm for the renal denervation system used in the present study. In that study2, RF treatment (30 seconds, 68°C target temperature, as in the present study) resulted in an average 89.5% reduction in renal norepinephrine when comparing treatment in one renal artery with no treatment to the contralateral renal artery at seven-day follow-up. Nerve immunostaining for tyrosine hydroxylase (marking catecholamine production) at seven days showed reductions in staining intensity. Observations of nerves without pathologic changes having very low tyrosine hydroxylase staining intensity were consistent with these nerves having been thermally damaged at one or more other sites along their length but not in the plane of section.
There has been only one preclinical study on a renal denervation system (Symplicity™; Medtronic, Minneapolis, MN, USA) previously published3. With the therapeutic failure of this system in a recent large randomised controlled clinical trial4, it is reasonable to focus on the key question of whether or not systemic energy transfer is at a sufficiently high level to achieve lasting efficacy while remaining safe. Developing sound surrogate efficacy measures in the porcine model and combining these with the comprehensive safety evaluation incorporated in the present study are the next steps in preclinical evaluation to prepare optimally for clinical trial.
Conclusion
The Vessix 7 Fr Renal Denervation System was found to be safe, operated as intended for human use in this porcine model (approximately 1 watt power, 30-second duration, 68°C temperature), inclusive of overlap treatment as a realistic worst case for both induced thermal and mechanical injury.
| Impact on daily practice Safety of the bipolar Vessix™ Renal Denervation System for radiofrequency ablation of renal artery nerves was investigated in normotensive healthy common swine, a well-characterised model for evaluating vascular safety. This study demonstrated the safety of the system during full artery length treatment, including overlapping treatment segments in the renal artery. Thermal injury healing over 180 days with segmental transmural medial fibrosis, complete endothelial recovery, no evidence of thrombus, and no injury to non-target organs adjacent to the treated segments was evident. Nerves adjacent to the treated segments showed thermal injury and fibrosis up to 180 days. Therefore this study demonstrates the safety of the device for renal denervation and provides evidence of nerve injury, but does not demonstrate efficacy of the treatment. |
Acknowledgements
The authors thank Elizabeth Davis of Boston Scientific for her assistance in preparing the manuscript.
Funding
Funding was from Boston Scientific Corporation.
Conflict of interest statement
G. Wilson is a consultant to, and D. Winsor-Hines, R. Tunstall, L. Davis, J. Bankes and B. Huibregtse are employees of Boston Scientific Corporation. P. Wilson, S. Hawley, M. Eskandarian and G. Svajger have no conflicts of interest to declare.