The following section of this supplement concerning current technologies focuses on the six CE-marked devices for renal denervation. The pace at which next-generation catheters are currently being designed is incredible and the fact that the field of renal denervation promises to be a highly profitable business has encouraged up to 65 companies today into positioning themselves in this emerging market. Multiple treatment modalities are being tested with various energy sources, intravascular drug delivery and non-invasive as well as non-renal artery approaches. In addition, the manufacturers of the currently discussed CE-marked devices are already working on second and third generations of their existing products.
Following extensive animal work and pivotal clinical trials, several technical and procedural issues have emerged which could be improved. The subsequent interventional wish list is rapidly growing and includes, to name just a few: radial support; smaller –potentially 5 Fr– catheters; shorter procedure times; minimal need for device manipulation; one size fits all catheters in order to minimise procedural costs; the possibility to undo the treatment effect in specific populations; monitoring features to predict adequate denervation; proper imaging features to assess the extent of local endothelial damage. It will certainly take time to respond fully to all these wishes, and it will be a challenge to develop a device that carries all the features mentioned above. And finally, it will be individual patient characteristics and local anatomy that might require a tailored approach in which more specific features might become necessary.
As of the publication date of this supplement, there are six devices which are currently being approved for clinical use in Europe: all of them carry specific features and all of them have certain positive as well as negative points. We look forward to future innovations but, even more importantly, we look forward to the results of the pivotal studies currently being conducted with these first- and newer-generation devices.
Description
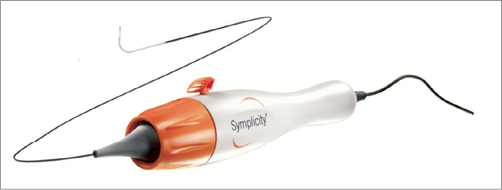
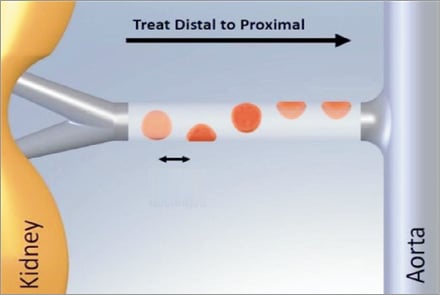
The current Symplicity renal denervation system is the result of the acquisition of Ardian Inc. by Medtronic in November 2010. The early 8 Fr guide compatible single electrode renal denervation catheter was improved and now fits through a 6 Fr introducer sheath. Although the currently used 2nd generation of the catheter is a non-over-the-wire system, a flexible tip allows an easy entry into the renal artery through a standard 6 Fr guiding catheter. The non-occlusive device uses a single unipolar electrode on the tip of the catheter to create a spot lesion using radiofrequency (RF) energy without cooling capabilities. The single RF electrode generates a temperature of up to 75 degrees at approximately 5 to 8 watts in two minutes per ablation. The device comes with a handle with which the operator can both rotate and flex the tip of the catheter. The latter is needed to manually steer the catheter in the renal artery through a 12 mm deflectable shaft and a self-orienting 5 mm flexible tip. The operator should aim to apply the energy in a helical pattern with a distance between treatment sites of at least 5 mm to avoid creating overlapping lesions. At least four ablations are proposed to generate sufficient sympathetic nerve denervation. Good wall contact is crucial; the device automatically shuts off after two minutes or immediately when either impedance or temperature exceeds programmed safety limits. Medtronic’s near-term pipeline includes a third generation of the device, which will feature a 6 Fr spiral over-the wire system with four unipolar electrodes that can be switched on simultaneously in a one-minute ablation to allow a faster and easier procedure.
Clinical data
A first-in-man study (Symplicity HTN-1) performed in Australia included 45 patients with therapy-resistant hypertension (i.e., systolic blood pressure >160 mmHg despite the use of at least three antihypertensive drugs, including a diuretic, and a renal function of >45 ml/min)1. Following initial success, the cohort was extended to a total of 153 patients. Mean procedural time was 38 minutes and the procedure proved to be safe with no major complications and four minor complications (one renal artery dissection and three access-site complications). Currently available three-year follow-up demonstrated an office-based blood pressure reduction of 31/16 mmHg. Following the open-label single-arm Symplicity HTN-1 study the Symplicity HTN-2 randomised controlled trial (n=106) was conducted and performed in Europe, Australia and New Zealand using the same inclusion and exclusion criteria. The safety of the procedure was demonstrated and the study demonstrated a significant drop in both systolic and diastolic blood pressure versus the control group at the six-month primary endpoint (–32/–12 mmHg vs. +1/0 mmHg p<0.0001 in the control arm)2. Recent presentation of two-year data demonstrated a sustained drop in office based blood prssure (–29/–10 mmHg). The programme was extended with the Symplicity HTN- 3 trial, a randomised trial with a sham control arm that is currently ongoing, a total of 530 patients will be enrolled. Furthermore, a large-scale all-comer registry (n=5,000) is currently ongoing and several small, pivotal but promising studies have been performed in patients with diabetes, end-stage renal disease, sleep apnoea, heart failure and tachyarrhythmias3-10.
Technical specifications
CE-mark: February 2008
Energy source: radiofrequency
Size: 6 Fr, one size fits all
Renal artery treatment range: ≥4 mm
Treatment time: 2 min/ablation, recommend minimum of 4 ablations per artery
Cooling features: no
Over-the-wire system: no
Total energy delivery: maximum of 8 watts
Maximum temperature: 75° degrees
Catheter length: 90 cm
Unique features
– 6 Fr compatible
– One size fits all
– Ability to select specific ablation sites in complex anatomy
Potential problem areas
– Need for manipulation with current version
– Longer procedure time due to the sequential activation of the electrode
– Risk of loss of vessel wall contact and subsequent incomplete ablation
Description
In 2004, CVRx was one of the first companies to enter the market with a device focused on nerve stimulation to alter haemodynamics. Compared to local (renal) sympathetic denervation, Baroreflex Activation Therapy is focused at the level of central sympathetic activity by directly stimulating the carotid baroreceptors. Baroreflex Activation Therapy reduces sympathetic nerve activity while increasing parasympathetic activity resulting in vasodilatation and a reduction of the systemic vascular resistance. The first generation Rheos system consisted of two leads and an implantable pulse generator. CVRx introduced the second generation Barostim neo™ system, which received CE mark in 2011. This new unilateral system requires a small, single-sided incision in the neck to gain access to the carotid artery where a 7 mm disc with a 2 mm electrode is placed on the carotid sinus. The lead is tunnelled to a small pocket where the pulse generator is placed. The therapy’s unique features allow it to be programmed to meet the individual patient’s needs. It is fully reversible and can be turned off and on to demonstrate patient response. The lead and implantable pulse generator are surgically placed subcutaneously, which can be performed under local anaesthesia. Total implantation time is approximately 1.5 hours in experienced hands.
Clinical data
After completing two feasibility studies (DEBuT and US Feasibility) the Rheos device received CE-mark approval in October, 200711,12. The four-year results of the DEBuT European feasibility trial of the Rheos system in patients with resistant hypertension demonstrated a drop in systolic blood pressure (office-based) of 53 mmHg and 30 mmHg in diastolic blood pressure13. A 322-patient randomised placebo controlled study with the Rheos device showed systolic blood pressure reductions of 35 mm SBP at 12 months and long-term follow-up (22-53 months) compared to pre-implant. Furthermore, significant reductions were noted in cardiac volumes and dimensions; the results show a clear regression of LV hypertrophy14,15. In a 30-patient study, the Barostim neo device showed systolic blood pressure reductions of 26 mmHg at three and six-month follow-up compared to pre-implant pressures16. Six of the 30 patients had previously been treated with renal nerve ablation. Results were similar to those patients not previously treated with renal nerve ablation. Barostim neo has received FDA approval to initiate a 310 patient hypertension study and a 60 patient Systolic Heart Failure feasibility study in the US.
Technical specifications
CE-Mark: Rheos: October 2007, Barostim neo: August 2011
Energy source: Electrical nerve stimulator
Size: small lead + generator of 72 mm/50 mm and 60 gram
Renal artery treatment range: N/A
Treatment time: implantation time of approximately 1.5 hours
Cooling features: N/A
Over-the-wire system: N/A
Total energy delivery: N/A
Maximum temperature: N/A
Catheter length: N/A
Unique features
– Can be switched on and off
– Central, multi-systemic mechanism of action
– Can be customised to meet the needs of the patient
– Can be used to treat patients who are not eligible for or who do not respond adequately to renal nerve ablation
Potential problem areas
– Risk of infection
– More complex procedure
Description
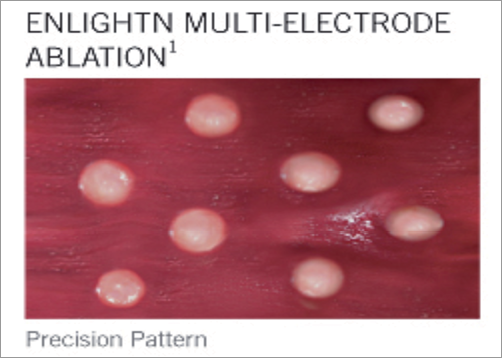
St. Jude Medical received CE mark for the first multi-electrode renal denervation system in December 2011. The system was designed to deliver a predictable pattern of four ablations with minimal catheter manipulations for a more efficient, easy and consistent procedure. The basket-designed catheter comes in two sizes, a small basket (16 mm treats 4-6 mm diameter vessels) and a large basket (18 mm treats 5.5-8 mm diameter vessels).
After introducing an 8 Fr sheath, an 8 Fr 55 cm guiding catheter is used to engage the renal artery. A selective renal angiogram needs to be performed in order to determine the device size. Both the 16 mm and 18 mm baskets are positioned in the renal artery without the use of a guidewire. The basket tip of the catheter is positioned just proximal to the renal bifurcation for the first or most distal treatment. Once positioned, the nitinol basket is expanded using the handle actuation. A diagnostic test is then performed to assure the activated electrodes have adequate electrode apposition. The system allows electrodes to be activated or deactivated as desired given anatomical or procedural considerations. Once apposition is confirmed, the ablation sequence is started by the operator. The generator automatically and sequentially activates the individual unipolar electrodes in 90-second intervals without the need to manipulate the device between ablations. The generator uses a maximum of 6 watts to initially reach the 75°C degrees target temperature for transmural lesions, then reduces power to maintain the target temperature over the duration of the ablation.
After the first round of ablations is completed, the operator collapses the basket and retracts the catheter approximately 1 cm and rotates the basket 45 degrees to offset the ablation pattern and to perform a second round of ablations. This positioning is designed to deliver longitudinal, circumferential coverage across the vessel.
Clinical data
St. Jude Medical commercially released the first-generation EnligHTN system in May 2012 after presentation of the pivotal first-in-man EnligHTN I trial (n=46). At one month, office based blood pressure reduction proved to be –28 mmHg systolic and –10 mmHg diastolic. Ambulatory systolic blood pressure reduction proved to be –10 mmHg versus –5 mmHg reduction in diastolic blood pressure17. Data through six months have been presented and confirm that the reduction is sustained18. An extended clinical trial programme is currently being created to incorporate a number of larger-scale trials, local registries and sponsorship of the EnligHTNment landmark clinical trial.
Technical specifications
CE-Mark: December 2011
Energy source: Radiofrequency (unipolar)
Size: 8 Fr, large and small baskets
Renal artery treatment range: 4 mm to 8 mm
Treatment time: 90 seconds/ablation, 8 ablations per artery
Cooling features: No
Over-the-wire system: No
Total energy delivery: Maximum of 6 watts
Maximum temperature: 75°C degrees
Catheter length: 115 cm
Unique features
– More predictable ablation pattern
– Potential to switch on and off the individual electrodes
– Minimal need to manipulate the device
Potential problem areas
– Potential need to use two different catheters
– Longer procedure time due to the sequential activation of the electrodes

Description
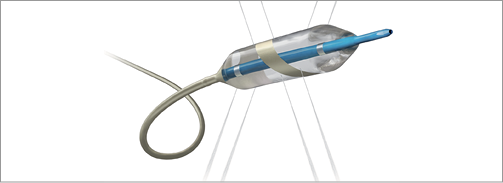
With the acquisition of Maya Medical in April 2012, Covidien entered the resistant hypertension market. The OneShot™ renal denervation system features an irrigated balloon catheter with a spiral electrode that ablates renal nerves via a unipolar radiofrequency energy source. Using a standard 0.014” guidewire, the low-pressure balloon can be inserted into the renal artery through a 7 Fr or 8 Fr guiding catheter (depending on the balloon size). Balloons are available with an electrode ablation length of 20 mm and diameter sizes of 5, 6 and 7 mm to treat vessels ranging from 4 mm to 7 mm. Eight tiny irrigation holes allow for an integrated cooling of the artery. Low-pressure irrigation runs continuously during balloon inflation with the goal of minimising overheating, endothelial damage and clot formation. A monopolar silver electrode wrapped on the balloon offers a standardised and reproducible ablation pattern. As the name already suggests, a single, two-minute ablation per artery is suggested as being sufficient, eliminating the difficulties in user variance when determining single ablation points. Two radiopaque markers on the catheter enable precise device positioning. One single ablation delivers 25 watts of RF energy and a maximum balloon temperature of 60 degrees.
Since the balloon size is fixed, one might need two different balloons per procedure in the case of different diameters observed in the left and right renal artery. There is some question as to whether tapering of the artery would conduct the energy to the point of least resistance. The truth of this hypothesis needs to be investigated further in upcoming clinical trial programmes.
Clinical data
Maya’s first-in-man experience was the Renal Hypertension Ablation System for chronic hypertension (RHAS) trial19, a small eight-patient study carried out in New Zealand between December 2011 and March 2012. No acute procedural complications nor serious adverse events were seen. At six months, the mean drop in systolic blood pressure was 42 mmHg versus 15 mmHg in diastolic blood pressure. Following the initial success, 11 additional patients were successfully treated at four European sites between May and June 2012 without serious adverse events. The larger multicentre RAPID trial, conducted in Europe and New Zealand, completed enrolment of 50 patients in February 2013.
Technical specifications
CE-Mark: February 2012
Energy source: radiofrequency
Size: 7 Fr to 8 Fr, balloon diameter 5, 6 and 7 mm.
Renal artery treatment range: 4 mm to 7 mm
Treatment time: one single 2-minute ablation per artery
Cooling features: yes
Over-the-wire system: yes
Total energy delivery: maximum of 25 Watts over length of electrode
Maximum balloon temperature: 60° Celsius
Catheter length: 74 cm
Unique features
– Single-treatment RF ablation per artery reduces procedure times
– Spiral electrode design offers standardised and reproducible ablation pattern
– Integrated irrigation cools the non-treated region of the artery
– Low pressure, balloon-based system delivered over a standard 0.014” guidewire
Potential problem areas
– Potential need to use two different balloons which might increase procedural costs
– Irrigation fluid flow might not be efficient when the balloon is fully expanded

Description
The Vessix™ Renal Denervation System was developed by Vessix Vascular (Laguna Hills, CA, USA), and acquired by Boston Scientific Corporation (Natick, MA, USA) in November, 2012. The Vessix™ Renal Denervation System consists of an over-the-wire balloon catheter and a radiofrequency generator. The non-compliant Vessix™ balloon is available in diameters of 4, 5, 6, and 7 mm. The exterior of the balloon is embedded with a helical array of electrodes to facilitate a consistent denervation treatment pattern; it is optimised to deliver thermal bipolar energy from the vessel lumen, outward, to a depth of a few millimetres.
Following the insertion of a 6 Fr introducer sheath through standard femoral access, a pre-treatment aortogram is performed with a standard 6 Fr pigtail catheter. The 6 Fr sheath is replaced with a longer (50 cm) 8 Fr introducer sheath to allow for passage of the renal denervation system. Additional selective angiograms performed on both renal arteries using a standard diagnostic or guiding catheter are recommended to ensure that the proper balloon size(s) are selected.
With the 8 Fr introducer sheath positioned at the front of the renal artery ostium, a standard 0.014”/0.018” guidewire is placed and the Vessix™ balloon is delivered over-the-wire. Following positioning within the renal artery lumen, the balloon is slowly inflated to 3 atm using a standard inflation device. Contrast medium may be administered to verify adequate apposition of the balloon against the vessel wall and the occlusion of the artery.
The radiopaque electrodes have independent temperature sensing and control features. The number of electrodes per balloon (4, 6 or 8) depends on the balloon size. Once balloon inflation is complete and the device is activated, the generator raises the electrode temperature to 68°C, the temperature at which treatment is conducted. The generator’s temperature-controlled algorithm, coupled with the simultaneous activation of all vessel-apposed electrodes, allows for the necessary depth of penetration to cause nerve denervation within 30 seconds. Electrodes with inadequate vessel wall contact are automatically deactivated by the generator. The bipolar electrodes ensure that the total amount of energy delivered to the artery is approximately 1 watt. In most renal arteries, one 30-second denervation cycle is sufficient for successful treatment, though longer arteries may require up to two treatment cycles.
Clinical data
The Vessix™ System received CE mark in April, 2012 based on preclinical studies and pathology data on 128 swine.
The Reduce HTN single-arm, international, multicentre post-market study has demonstrated a reduction in systolic and diastolic blood pressure of –23 mmHg/-13 mmHg at one month (n=34), –27 mmHg/–10 mmHg at three months (n=34) and –36 mmHg/ –14 mm at six months (n=6).
The REDUCE-HTN study completed enrolment of 150 subjects in April, 2013 to assess the safety and efficacy of the Vessix™ renal denervation system.
Technical specifications
CE mark: April 2012
Energy source: radiofrequency
Size: 8 Fr, balloon length 25 mm, balloon diameter 4, 5, 6 and 7 mm
Renal artery treatment range: 3 mm to 7 mm
Treatment time: 30 sec/ablation, 1 to 2 ablations per artery
Cooling features: no
Over-the-wire system: yes
Total energy delivery: maximum of 2 watts
Maximum temperature: 68 degrees
Catheter length: 90 cm
Unique features
– Very short procedure time
– Potential to switch on and off the individual electrodes
– Minimal need to manipulate the device
– Bipolar electrodes
Potential problem areas
– Potential need for two different balloons in a single patient
– Lack of cooling
– Need for 8 Fr long sheath


Description
With the Paradise catheter ReCor Medical introduced two new concepts in the field of renal sympathetic denervation: first, the use of ultrasound instead of radiofrequency energy and second, a circumferential denervation approach. The company has over 14 years of experience in developing catheter-based ultrasound devices. The Paradise system holds a cooled balloon catheter with a self-centred piezoelectric ultrasound transducer which enables controlled, uniform and circumferential energy delivery without direct wall contact. The ultrasound energy consists of high-frequency sound waves (rapid mechanical oscillations). The sound waves pass through the surrounding cooling fluid of the balloon and generate heat in the surrounding tissue with the goal of causing nerve damage.
Once standard femoral access is achieved, a selective renal angiogram using a standard guiding catheter allows the operator to measure the diameter of the renal artery. With this measurement, the operator can choose between a 6 mm or 8 mm balloon having the potential of covering arteries from 4 mm to 8 mm in diameter. The current version of the device is an over-the-wire system, which fits through a 7 Fr guiding catheter. The 10 mm long non-compliant balloon easily enters the renal artery and, once positioned, the generator auto-inflates the low-pressure balloon. The cooling fluid (sterile water) starts to circulate automatically and 30-second utrasound emissions can be delivered. In order to achieve an optimal denervation the operator typically delivers energy at three different spots in the renal artery.
Clinical data
The first-in-man South African REDUCE study tested the Paradise system in 15 patients with therapy-resistant hypertension20. The device proved to be safe with no device-related procedural adverse events. Six-month results showed an office-based blood pressure reduction of –29/-15 mmHg (n=15). Home-based blood pressure reduction was shown to be –13/–9 mmHg in systolic and diastolic blood pressure respectively (n=14). Ambulatory blood pressure reduction was –16/–9 mmHg in systolic and diastolic blood pressure, respectively (n=13). All changes at 6 months were statistically significant. Systematic follow-up imaging was performed, and in two patients treated with suboptimal cooling flow rate, signs of newly acquired renal artery stenosis were observed. The cooling flow rate was consequently increased in order to resolve the problem. The final results of the REDUCE trial are scheduled for late 2013. At present, two non-randomised studies are ongoing. In France, the 20-patient REALISE study is completing enrolment. Additionally, the post-market multicentre European ACHIEVE study will enrol a total of 50 patients with therapy resistant hypertension.
Technical specifications
CE-Mark: Q4-2011
Energy source: ultrasound
Size: 6 Fr, balloon diameter 6 mm and 8 mm (5 and 7 mm balloons upcoming).
Renal artery treatment range: 4 mm to 8 mm
Treatment time: 30 sec/ablation, up to three ablations per artery
Cooling features: yes
Over-the-wire system: yes
Total energy delivery: 12 watts
Maximum temperature: 25 degrees inside balloon
Catheter length: 85 cm
Unique features
– Intravascular cylindrical ultrasound transducer
– Balloon with high flow rate, closed-circuit cooling system
– Circumferential denervation pattern
Potential problem areas
– Arteries need to be properly sized in order to prevent balloon over-inflation
– Potential need for two different balloon catheters in the same patient
– Risk for restenosis due to circumferential ablation pattern