Abstract
Aims: The morbidity and mortality of surgical aortic valve replacement are increased in elderly patients with multiple high risk comorbid conditions. Percutaneous prosthetic aortic valve replacement (PAVR) via the femoral arterial approach is feasible in selected patients, who are poor operative candidates, with satisfactory short term outcomes. It is conceivable that patients with poor LV function may benefit from periprocedural cardio-circulatory support. We evaluated the short-term safety and efficacy of using the TandemHeart® PTVA® System (p-LVAD) to deliver extracorporeal circulatory support in patients undergoing PAVR.
Methods and results: Between April 2006 and May 2007 the TandemHeart® was used in 10 patients (age: range 64-85, median 80) undergoing elective PAVR using the CoreValve™ Revalving System. The median (range) time for implementation of circulatory support was 32 (22-40) minutes. A pump flow up to 4.6 L/min was achieved. Systemic haemodynamics were maintained in all but one patient. The median (range) systemic arterial pressure (MBP) was 77 (67-89) mmHg at baseline and 76 (61-91) mmHg after pump functioning. A major systolic blood pressure drop (systolic blood pressure < 70 mmHg, pulse pressure < 10 mmHg, occurred in one patient due to PAVR related pericardial tamponade. Median (range) duration of support was 64 (60-93) minutes. Successful weaning was achieved in all patients. There was one in hospital death. Survival at 12 months was 90%, at 15 months 70%. Vascular access site complications were seen in two patients. One patient suffered a mild to moderate access site bleeding, one a local wound infection. There was no technical device failure.
Conclusions: The TandemHeart-PTVA® may provide a valuable safeguard during high risk PAVR procedures and enables precise delivery of the CoreValve prosthesis. The rate of device related cardiac and vascular complications was acceptable.
Introduction
Degenerative aortic stenosis (AS) is a frequent heart valve disease in Western countries, of which the prevalence steadily increases with age1,2. Open heart surgery with mechanical or bioprosthetic valve replacement is the reference standard therapeutic approach for patients with severe aortic valve disease, offering symptomatic relief and improving long-term survival in most patients. The Euro Heart Survey, however, revealed that one-third of elderly patients with severe, symptomatic AS are not referred or declined for surgery by the attending practitioner2. This is particularly the case for very elderly patients, patients with reduced left ventricular (LV) function and/or associated comorbid conditions. Since the prognosis of medically treated patients and those who underwent ‘plain’ balloon valvuloplasty is poor3,4, a less invasive techniques for treatment of high-risk patients such as PAVR may be an alternative. The CoreValve Revalving™ System (Medtronic, Minneapolis, MN, USA) consists of a porcine valve mounted in a self expanding nitinol frame that is implanted by slowly pulling back a protective sheath. The implantation technique allows delicate and minute adjustments for correct valve positioning5-7. Initially, with the introduction of the PAVR technique, the clinical protocol recommended the use of mechanical circulatory support (MCS) during implantation to maintain adequate systemic circulation. This was realised by the use of femoro-femoral cardiopulmonary bypass (CBP).
To reduce the surgical trauma, to render PAVR truly percutaneous and to increase patient comfort, we started using the TandemHeart®, which is a Percutaneous Transseptal Left Ventricular Assist Device (PTVA®) system (CardiacAssist Inc., Pittsburgh, PA, USA) instead of CBP. This system allows for rapid implementation of circulatory support, delivering up to 4,5 litres of blood flow per minute, using standard interventional techniques in the catheterisation laboratory8. We report the Rotterdam single centre expertise with assisted circulation using the TandemHeart® PTVA System during PAVR. We performed a post hoc analysis of prospectively (on PAVR procedures9) collected data.
Patients and methods
Patients
From April 2006, the TandemHeart® percutaneous circulatory support was used in a series of 10 consecutive patients treated with the CoreValve Revalving™ System (CRS) in the Thoraxcentre, Erasmus University Medical Centre, Rotterdam (The Netherlands). Patients were considered for this analysis if a clinical follow-up of 12 months or more could be established.
Patients were considered for PAVR provided: 1) a symptomatic severe stenosis (valve area < 1 cm2, 0.6 cm2/m2 as by echocardiographic measure) of the native aortic valve, 2)10,11 reflecting a high peri-operative risk and 3) a contraindication to surgery because of concomitant comorbid conditions assessed and agreed to by both an independent cardiologist and a senior cardiovascular surgeon. Exclusion criteria for the PAVR study protocol included: hypersensitivity or contraindication to aspirin; heparin; thiënopyridines; nitinol or contrast media that could not adequately pre-medicated; sepsis or active endocarditis; excessive femoral, iliac, or aortic atherosclerosis; calcification or tortuosity; aortic aneurysm; bleeding diathesis or coagulopathy. Any condition considered contraindicated to extracorporeal assistance. This study was approved by the local Medical Ethics Committee, and all patients and their closest relatives signed informed and written consent.
Percutaneous valve implantation procedure
The technique of PAVR using the CoreValve™ Revalving System has been described in detail elsewhere.6,7,8,22 In the present series of 10 patients, the 21 Fr revalving system was used in the first three patients, while the 18 Fr system was used in the remaining six patients. Vascular access, via the common iliac artery or common femoral artery, was obtained by surgical cut-down in four patients, and echo-guided vascular access was obtained in six with the use of vascular ‘pre’-closing device (a 10 Fr Prostar XL). A 5 Fr pigtail was put in place via the left radial artery into the ascending aorta for pressure recording and angiography to guide valve positioning and assessment of the final result. Clinical and haemodynamic outcomes were assessed serially during the procedure. Valve implantation was performed under general anaesthesia in four and dissociated anaesthesia in six (sedation and analgesia, but no intubation and ventilation).
The Tandemheart (insertion technique)
The Tandemheart®PTVA® (CardiacAssist, Pittsburgh, PA, USA) (Figure 1a) incorporates arterial perfusion cannula configurations ranging from 9 to 17 Fr, an unique 21 Fr venous transseptal cannula, and a centrifugal blood pump (Figure 1b).
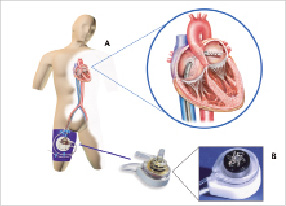
Figure 1. The Tandemheart®PTVA® incorporates arterial perfusion cannulae, a 21 Fr venous transseptal cannula, and a centrifugal blood pump.
Oxygenated blood from the patient’s left atrium is supplied to a centrifugal pump by the transseptal cannula and than returned to the patient’s systemic circulation. The pump connects to a microprocessor-based controller that displays PTVA speed and flow. These parameters are controlled by adjustment of a single knob. A standard transseptal puncture technique, using an Inoue wire, was used to gain access into the left atrium from the right femoral vein. Transseptal puncture was carried out by an experienced operator only after clear delineation of the inter-atrial septum using ICE (Intra Cardiac Echocardiography, ACUNAV, Siemens, Germany). Details on the of the Tandemheart circulatory support and implementation are reported elsewhere12. At the moment of valve crossing with a straight Kimal wire, the Tandemheart was tuned down to a stand-by-mode. The stand-by-mode entails the reduction of the RPM to the lowest RPM value possible (± 3500 RPM) resulting in a minor to insignificant circulatory support. A fully active pump may reduce the already impaired leaflet motion, and enhance the difficulty of crossing the stenotic valve. At the moment of balloon valvuloplasty and CoreValve™ positioning and placement, the pump was returned to full circulatory support. Weaning from the Tandemheart was performed in a stepwise protocol, with progressive reduction of pump assist, by reducing pump speed from 7500 to 3500 rates per minute (Pump flow +/- 400 ml/min), and adapted to the medical condition of each patient individually. The pump was not stopped until immediately before removal. The final removal decision was based on medical judgement.
Anticoagulation
A constant flow (900 units/hr.) of heparinised infusate is maintained, thus providing a localised concentration of heparin in the interior of the pump in order to obtain localised anti-coagulation, thereby minimising systemic heparinisation, the risk of bleeding and thrombus formation. During the PAVR, the patients received weight adjusted intravenous heparin to achieve an activated clotting time of 300 to 350 seconds for the duration of the procedure. Post-implantation, a dual antiplatelet strategy of aspirin 75-160 mg and Plavix 75 mg, each daily, for six months, followed by aspirin 75-160 mg, indefinitely, was prescribed.
Statistical analysis
Categorical variables are described as counts and percentages. Continuous variables are expressed as median and quartiles. Statistical significance was achieved at p<0.05. The insertion time was defined as the time from providing of the transseptal puncture needle to the ‘full’ heparinisation following connection of the femoral cannula to the pump. The duration of support was defined as the time from ‘full’ heparinisation at time of connection to the pump till the removal of the femoral cannula. Statistical analysis was performed by Cardialysis, Rotterdam (The Netherlands) with SAS 8.2 software (SAS Institute Inc, Cary, NC, USA).
Results
From April 2006 to May 2007, 10 symptomatic patients (five men, five women; median age 80 years; range 64-82) had a PAVR supported by the Tandemheart®. Baseline characteristics are listed in Table 1.
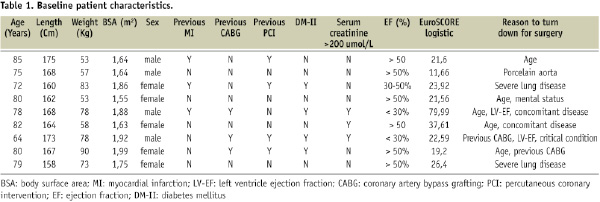
The median (range) calculated logistic EuroSCORE was 22.59 (range 21.56 to 26.40).
The left ventricular systolic function (echocardiographic estimated ejection fraction, EF) was reported good (EF > 50%) in six, moderate (EF 30-50%) in two and poor (EF < 30%) in another two patients. The patient risk in our series was mainly driven by age, renal function and EF (Table 1).
Implementation of circulatory support was successful in all patients. Median (range) time for TH® implantation was 32 (22-40) minutes. The median (range) duration on circulatory assist was 64 (60-93) minutes. The median (range) systemic arterial pressure (MBP) was 77 (67-89) mmHg at baseline and 76 (61-91) mmHg after pump functioning. A major systolic blood pressure drop (systolic blood pressure < 70 mmHg, pulse pressure < 10 mmHg, occurred in one patient due to pericardial tamponade following crossing of the valve with a straight Kimal wire. A needle pericardiocentesis was needed. A valve prosthesis was nevertheless correctly implanted. The patient died at day six due to severe sepsis and end-organ (renal) failure. An iliac artery rupture occurred in one patient during revalving. The procedure was aborted, the TandemHeart® was weaned and the patient was sent for surgical vascular repair and had a further uneventful hospital recovery. The latter patient was excluded from further outcome analysis in our series. Overall procedural success was 78% (Table 2).
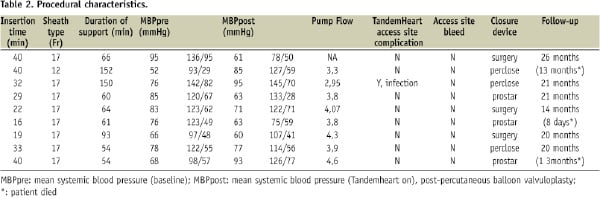
The final aortic regurgitation rate was < 2 in all patients. There was no neurologic event nor any need for permanent pacing reported in this patient series.
The median (range) clinical follow up was 585 days (405-628). As already indicated, there was one in hospital death at eight days following PAVR. Survival at 12 months was 90%, at 15 months 70%. Mild access bleeding occurred in one patient, local wound infection in another. Local homoeostases was provided by a vascular pre-closure device (Prostar®XL) in both these patients.
Discussion
This is the largest report investigating the haemodynamics and outcomes of patients undergoing PAVR with the Tandemheart. The main results of this study can be summarised as follows: The TandemHeart® PTVA® System could easily and quickly be implemented by an experienced operator using intracardiac echocardiography, the procedure and Tandemheart related complications were low. The Tandemheart provided a valuable safeguard during PAVR and allowed for precise positioning of the CoreValve prosthesis in a high risk population. The one year mortality in this high risk patient population was relatively low. Vascular access was performed in a “true percutaneous fashion” in the majority of patients with the aid of vascular ‘pre’-closing device. An ultrasound-guided transseptal puncture technique, integrating different imaging modalities available in the catheterisation suite, intracardiac echocardiography (AcuNav ICE, Siemens Medical Systems, Mountain View, CA, USA), was tested and implemented in this patient series may provide excellent therapeutic applications in other, ‘complex’ anatomical settings.
Transvascular, retrograde implantation of aortic heart valves is an emerging and promising technology that may benefit patients with high risk features for surgery. The experience with these systems continuous to grow, with leading centres and investigators contributing meaningful information toward the application and development of the latest technologies. Device and procedural enhancements are required to assure reliable and safe prosthesis delivery, positioning, deployment, anchoring, function and durability. We introduced the concept of ‘stand-by’ circulatory support during native valve crossing and re-activation during correct CoreValve™ positioning. The latter is crucial, not only for its proper function but also in respect to mitral valve function and preservation of coronary flow13,14. Cardiac motion and (trans-) aortic flow may impede precise positioning and expansion of the valve.
Also during balloon valvuloplasty, prior to device placement, we prefer MCS (mechanical circulatory support) over rapid ventricular pacing (RVP) of the right ventricle as advocated by other operators5. In case of adequate capture burst pacing at a rate of 220 min-1 will sufficiently lowers systemic blood pressure and (trans-) aortic flow but only provides a limited time window to position and expand the stent without any room for adjustments. RVP might obliterate the left ventricular outflow track and make positioning of the valve impossible. Moreover, rapid ventricular pacing, may induce asystole or malignant ventricular arrhythmia, which occasionally may lead to refractory haemodynamic collapse, especially in patients with structural heart disease as result of age or long lasting pressure overload15,16. MCS allows titration to the haemodynamic needs of individual patient and the stage of the procedure. It may reduce the risk of global ischaemia and death in a very high risk patient population.
For reasons of logistics, safety and efficacy, we prefer the TandemHeart® PTVA® System to deliver extracorporeal circulatory support to provide a preserved right ventricular and pulmonary function, rather than full CPB or related systems. However, this requires transseptal puncture technique. Direct visualisation of the inter-atrial septum by intracardiac ultrasound may improve safety and increase efficacy of this procedure17,18. In experienced hands, circulatory support up to 4.5 l/min. can be provided in less than 30 minutes. Centrifugal flow provides for ease of set-up and operation (no synchronisation) by the cardiology-intervention team without requiring the assistance of a full trained perfusionist. The flow rates provided by the device might even be sufficient to prevent, and even reverse, organ dysfunction in cardiogenic shock patients19. As opposed to other modalities of percutaneous circulatory support (CPS) used in this indication20,21, the Tandemheart system keeps the patients lungs as its own ventilator, and may be used to support patients for a longer period of time without major haematological or pulmonary complications or haemolysis12. The device was well tolerated by the awake patient. No adverse effects were observed during the use of the Tandemheart other than vascular access site complications in two patients (minor bleeding, n=1; local wound infection, n=1). It is unclear whether this was related to the extra vascular access needed for the p-LVAD, the PAVR or the percutaneous closure of the femoral vessels. The device was considered lifesaving in one of our patients at the moment of a PAVR procedure related acute cardiac tamponade. The preload to the left atrium, and the pump, at that time was preserved by volume loading and increasing the heart rate. A needle pericardiocentesis was requested. Survival at one year in our series was 90%, as compared to 87% for the overall patients treated with AVR and 62% for the patients treated with PAVR in our centre in the same time period22. These competitive results compare favourably with the pre-procedural median logistic EuroSCORE, and even to surgical reports of high risk patients undergoing AVR23,24.
Several important limitations of this study should be acknowledged. First, the present investigation was performed at a single experienced centre; a multicentre study is required to more fully understand the generalisability of the present results. As to this time of development, in most patients PAVR is feasible without CPS. However, the Tandemheart should still be considered in some high-risk patients, particularly in those who present with acute heart failure and/or that need concomitant (high-risk) PCI. Even in patients with combined valvular and complex coronary artery disease, those that are poor candidates for PAVR, the Tandemheart may fit into a hybrid treatment approach25. More detailed haemodynamic studies may help to identify those patients that potentially benefit the most of assisted circulation during aortic revalving procedures. At all times the haemodynamic benefit related to implementation Tandemheart® support should be weighted against potential risk involved, with a special focus on risks related to placement of the transseptal and the arterial cannula. In analogy with the literature, we used the logistic EuroSCORE for perioperative risk calculation in PAVR. However, the logistic EuroSCORE might overestimate mortality risk in this patient group. It may be clear that in the future that more appropriate risk models will be needed.
Conclusion
The elective use of the Tandemheart for circulatory support during PAVI procedures preserved haemodynamic stability in our patients, regardless of the intrinsic cardiac function, and allowed for a precise delivery of the self-expanding, nitinol framed, CoreValve prosthesis.

