Abstract
Aims: Very late stent thrombosis (VLST; >1 year) is an infrequent but potentially serious complication, whose risk factors have not been fully elucidated. This investigation sought to develop a clinically useful risk stratification score for VLST following drug eluting stent (DES) placement.
Methods and results: A Cox proportional hazards multivariate model of VLST was developed based on follow-up into a second year of patients enrolled in the ARRIVE registries, utilising readily available baseline clinical and angiographic characteristics. ST predictors between one and two years were identified among 7,459 consecutively enrolled patients who received a TAXUS® Express2™ (Boston Scientific, Natick, MA, USA) DES. Six significant predictors were found: presence of renal disease, prior myocardial infarction, multiple stenting, bifurcation lesions, prior CABG, and smoking at baseline. Each predictor was assigned a score, then summed for a maximum possible score of 10. Stratification into low and high risk groups revealed that VLST developed in 0.5% of 6,759 patients with scores <5, and 2.6% of 700 patients with scores ≥5.
Conclusions: We defined a VLST risk score for patients during the second year post DES-placement that provides a useful tool for risk stratification.
Introduction
The introduction of drug eluting stents (DES) has significantly reduced the need for repeat interventions compared to bare metal stents (BMS). However, concerns regarding increased late stent thrombosis events and late term mortality compared to BMS were raised at the 2006 European Society of Cardiology in Barcelona1, which contributed to reduced utilisation of DES worldwide. Although very late stent thrombosis (VLST) is infrequent, it may be associated with potentially catastrophic consequences including myocardial infarction and death. The Swedish SCAAR group initially reported an increased rate of MI, death and ST with DES after six months, but have subsequently published more extended data showing no difference in these endpoints2. Stone and colleagues reported that although the risk of very late stent thrombosis (assessed with the protocol definition excluding ST events that followed repeat revascularisation) was higher in patients receiving a DES versus a BMS, the rates of death and myocardial infarction did not differ between the two groups through four years, and the rates of target-lesion revascularisation were markedly reduced with DES3. None of these studies, however, have had sufficient power to elucidate which of many potential factors are most closely related to the risk of developing ST, including procedural, lesion and patient factors, as would impact the outcomes of studies enrolling various types of patients and lesions4.
We previously reported a clinically useful risk score for stent thrombosis (ST) in the first year following DES implantation developed with data from the TAXUS ARRIVE 1 and 2 stent registries5. With the availability of longer term (two year) data from the ARRIVE registries, we sought to develop a score predictive of very late ST (VLST, >1 year).
Methods
The goal of this study was to use statistical modeling to develop a simple risk score for definite/probable VLST as defined by the Academic Research Consortium (ARC)6. Registry data from the ARRIVE program were used as the source to develop the risk score model. The ARRIVE program is a two-part prospective “real-world” registry program undertaken in conjunction with the FDA to study usage patterns and long-term outcomes of the TAXUS® Express2™ stent in the United States. ARRIVE 1 enrolled 2,487 patients, while ARRIVE 2 enrolled 5,005 patients; thus, data from over 7,000 patients were used in developing this risk score model. All analyses were conducted using statistical software SAS 8.2 (SAS Inc., Cary, NC, USA).
Data collection, endpoint definitions and follow-up
The ARRIVE registry is described in detail elsewhere7. Briefly, this was an all-comers, consecutively enrolled, real-world post-market registry of the TAXUS Express stent in the USA. No specific inclusion-exclusion criteria were mandated, and each patient was enrolled at procedure initiation to minimise potential bias by exclusion of complicated or unsuccessful procedures. All cardiac events including ST were adjudicated by an independent external clinical events committee. Patients were followed up at 1, 6, 12 and 24 months. Data quality was ensured by independent audits of 100% of all cardiac events including ST, and random audits of the remaining data in 20% of ARRIVE 1 and 10% of ARRIVE 2 patients.
Thirty-nine candidate variables were considered based on clinical relevance and prior research (Table 1)5,8.
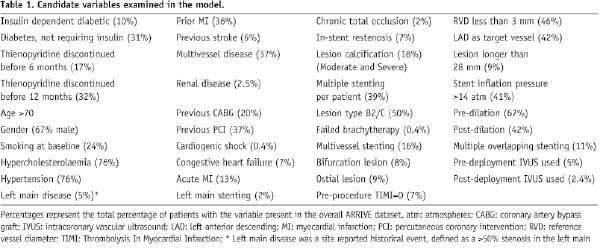
To understand the impact of each of these individual variables on VLST, a Cox univariate regression analysis was performed (Table 2).
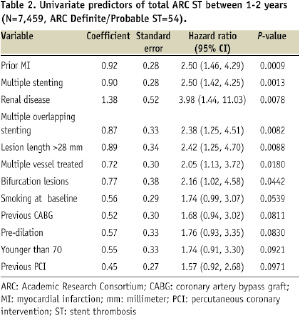
The initial list of 39 variables was narrowed to 15 variables with a P-value <0.1. A value of 0.1 was used to narrow the list without losing any potentially significant variables for inclusion in the second part of the regression. These covariates were then entered in a multivariate Cox regression model to examine and adjust for multiple covariates. Backward selection was used with a threshold to stay in the model set at 0.1.
The resulting model yielded six significant (P<0.05) preliminary independent variables predictive of VLST. Included in this list of six preliminary variables was treatment for failed brachytherapy. Sensitivity analysis was performed on the model to assess the impact of brachytherapy, since brachytherapy is now rarely used in the United States. Prior brachytherapy was removed from the list of initial variables, and ISR stenting entered the model, while the remaining five were constant. The C-statistic and goodness of fit testing were similar. Since patients with ISR stenting had a high degree of overlap with patients with prior brachytherapy, another analysis was performed after removal of the patients (rather than just the variable) with prior brachytherapy from the dataset. Four prior variables remained in the predictor list, and two additional predictors entered: prior CABG and bifurcation lesions. The C-statistic and goodness of fit testing were not impacted significantly. Consequently, the final model was prepared omitting brachytherapy patients (N=33) from the analysis, of which two (6%) had VLST.
The resultant final model yielded six statistically significant variables. Using the statistical modeling guideline by Hosmer & Lemeshow9, which estimates one variable per 10 cases, one would expect five to six variables in the final model. Each variable was assigned a score equal to twice (two times) its coefficient (i.e., natural log of the corresponding hazard ratio) rounded to the nearest whole integer, then summed to yield a maximum possible VLST score of 10. The coefficients of these risk factors were used instead of the hazard ratios themselves, to minimise the influence of the most powerful variables. To evaluate the goodness of fit of the model, we report the C statistic (area under the receiver operator characteristic curve) and Hosmer-Lemeshow goodness-of-fit statistic (high C-statistic and low [not significant] P-value corresponds to good fit). A bootstrap method was used as a validation tool to assess the model stability, and results from this bootstrap simulation analysis were able to confirm the predictors identified in this model.
Results
In the ARRIVE program, 56 patients (0.75%) had VLST during the second year post-DES placement. Variables initially found to be predictive of VLST included treatment for failed brachytherapy. Treatment for failed brachytherapy was initially the strongest predictor of VLST, yet present in only 0.4% (33/7492) of the total patient population, of which only 2/33 (6%) had VLST. However, coronary brachytherapy is now used infrequently, potentially limiting the utility of the model in current clinical practice. Therefore the definitive analysis was performed with the patients having failed brachytherapy removed. This resulted in the final number of patients included in the analysis of 7,459, with 54 of those patients having had a VLST (48 definite VLST, and six probable VLST events according to the ARC definition).
The final model thus included the following variables which were predictive of VLST: the presence of renal disease (site reported, defined as serum creatinine >3 mg/dl or patient on dialysis), prior history of myocardial infarction, multiple stenting (includes same vessel, overlapping and multivessel), bifurcation lesions, prior CABG, and baseline smoking. Integer values of 1-4 based on a rounded multiple of each factor’s coefficient were assigned, yielding a maximum possible score of 10 (Table 3).
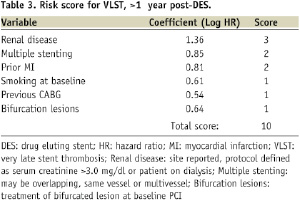
The average risk score of patients with ARC definite/probable VLST was 3.4±1.9 versus the average score in those without VLST, 2.1±1.6, P<0.0001.
When all ARRIVE patients are ordered by increasing score, there is an upward trend in the risk of developing VLST (Figure 1).
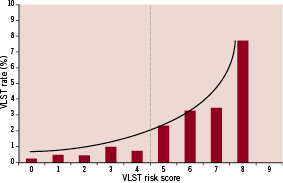
Figure 1. VLST and individual risk scores. Distribution of VLST by individual risk score in the overall ARRIVE population.
The area under the curve (AUC) or C statistic, a measure of discrimination of the model, was 0.7 indicating moderate discrimination between patients with ST versus those without (Figure 2).
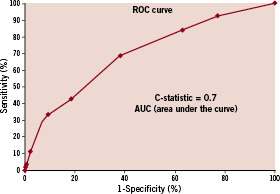
Figure 2. ROC Curve - Model performance was assessed by examination of the receiver operating characteristics (ROC) curve.
The P-value from the Hosmer-Lemeshow goodness of fit test was 0.5, indicating a reasonable fit.
Finally, all ARRIVE registry patients were divided into either a low or high risk group using the risk score. The groups were allocated empirically based on the distribution across the total possible individual scores (Figure 1). The ST risk curve was fairly flat in patients with scores 0 through 4. The majority of patients (91.0%, N=6,759) were in the lowest risk group (<5 points), with an ST rate of 0.5%. The remaining 9% (N=700) were in the high risk group (score 5-10 points) with a 2.6% risk of developing VLST (Figure 3).
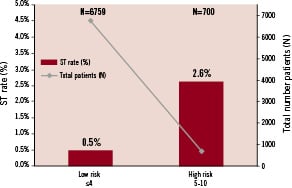
Figure 3. Risk Stratification - Risk stratification of patients in ARRIVE reveal very few patients in the highest risk category for developing VLST.
Discussion
This analysis defined a tool for the clinical assessment of VLST risk among patients who had undergone DES implantation, by using six readily available clinical and angiographic variables that predicts a 5-fold variation in the risk of second year VLST (0.5 to 2.6%). This score may help identify patients who are likely to be at a higher risk for developing VLST, and who may benefit from prolongation of dual antiplatelet therapy beyond one year. The fact that the majority of these “real-world” patients fall into the lowest risk category (2nd year VLST 0.5%), while less than 10% are in the high risk category (2nd year VLST 2.6%), is somewhat expected considering that the overall rate of VLST is so low.
Despite the fact that the ARRIVE registries enrolled a large number of complex patients and lesions, with the majority of patients (64%) receiving DES in an off-label indication, the overall 0.7% rate of stent thrombosis was low, although it was higher (1.0%) in expanded-use patients8. This dependence of ST rate on complexity is similar to the data reported by Win and colleagues in the EVENT registry, in which the investigators demonstrated that off-label use of DES is associated with higher rates of stent thrombosis11.
The importance of this analysis, however, is that it provides greater distinction than simple on-label vs. expanded use categorisation. It is thus similar to our previous risk score for first year ST, which was built on the hazard ratios of multivariable predictors including discontinuation of thienopyridines by six months as the strongest predictor. In contrast, discontinuation of thienopyridines by either six or 12 months is not a predictor of VLST in the current analysis, although this may have been biased by differential utilisation of prolonged thienopyridine treatment in various risk groups. Recent data indicate that continuation of clopidogrel therapy beyond six months results in a lower incidence of death or MI12, especially in patients with diabetes13. Investigators in the LAST study specifically reported that no cases of late ST were observed in patients taking dual antiplatelet therapy14. However, an observational study of post-DES patients through three years by Park and colleagues15 found that continuation of clopidogrel beyond one year did not appear to reduce late stent thrombosis or clinical events. Airoldi also found in a long-term study of over 3,000 patients, that there was no association between stent thrombosis and termination of antiplatelet drug use after six months16, although an elevated hazard ratio for ST was seen with cessation of dual antiplatelet therapy during the first six months.
Current ACC/AHA guidelines for the management of PCI patients recommend extending dual antiplatelet therapy (aspirin and thienopyridine) to at least one year post-DES in patients who are not at an increased bleeding risk17. The benefits of extending thienopyridine therapy in stented patients will be addressed by the large, multi-sponsor DAPT (dual antiplatelet therapy) study, which will compare 30 months of DAPT to 12 months of DAPT in patients who are free of significant clinical events during the first 12 months after bare metal and drug eluting stents18.
It is unclear whether the substitution of prasugrel as an antiplatelet agent will reduce the risk of ST, particularly VLST events when used in DES patients. Results from the TRITON TIMI 38 trial of patients with acute coronary syndromes demonstrated a statistically significant decrease in ST rates through 15 months and also between 30 days and 15 months in patients taking prasugrel compared to those taking clopidogrel19,20. However, longer-term data are not yet available. Prolonged prasugrel use may reduce the incidence of VLST events. Alternatively, the overall incidence of VLST may be unchanged by prasugrel due to differences in the pathophysiology of very late ST events. In a review of ST events in ARRIVE, Lasala et al8 noted that 23.1% of patients with VLST were still taking both aspirin and a thienopyridine, and 54% were taking at least one antiplatelet medication. Interestingly, Damon and colleagues also found that 23% of patients with late ST events were still taking DAPT, and in 51% taking at least one antiplatelet medication21, and further suggested that very late ST events are pathologically distinct from early ST events.
Stratification of patients based on risk of developing late ST is difficult due to the low event rate. Several studies have identified predictors of ST6,8,10, but none has identified a useful or validated score for the purpose of identification of VLST risk. Recently, investigators from the DERIVATION study published an ST risk score that they applied to early, late, and very late ST rates, but its application in “real-world” settings remains unknown, as it was an observational, single-centre study and it suffers from a low total number of patients available with low-frequency events such as VLST10.
There seem to be important differences in the models for ST in the first versus in the second year. In our earlier model for ST risk in the first year, risk factors were related more to thienopyridine compliance and to clinical factors (long lesions, small vessels, diabetes) already known to increase the risk of ST in both DES and BMS patients. In the current model, stent thrombosis events beyond one year seem to be less related to medication compliance (or use of dual antiplatelet therapy), and more related to clinical markers of patient complexity (prior MI, prior CABG, or renal failure). Moreover, while most cases of VLST in this analysis were ARC definite (N=48) and may be related to late endothelial coverage of the DES struts22,23, it is unknown whether progression of background natural history events in diseased but non-stented segments of the artery might also play a role in VLST events. It is therefore possible that extended thienopyridine therapy or use of a more uniformly potent agent such as prasugrel, may also offer protection against clinical events due to both causes.
We identified renal disease as a predictor of VLST, but not of ST in the first year, which is in agreement with findings of Park and colleagues, who also noted that renal disease was predictive of late ST beyond 30 days, but not early events26. We also found smoking at baseline and multiple stenting to be predictive of VLST, as it was of ST in the first year.
Limitations of the current risk score include the relatively small number of events which limits the discriminatory ability of the model. Although data used in creation of this score came from a “real-world” registry of over 7,000 patients, the number of patients with a VLST was still relatively small (n=54). Furthermore, this model was developed from a data set which included only one type of DES, the TAXUS Express paclitaxel-eluting stent, so the data may not necessarily be applicable to other DES as it is possible that different DES might be associated with different ST risks. Despite the fact that the model yielded statistical significance, its clinical impact has yet to be measured. Also, this model should have further validation in other large data sets before it is used to guide clinical decisions of how to treat patients following DES. This model attempts to further define those patients in whom the physician may want to extend the use of DAPT. However, it is important to weigh the risk/benefit of possible increased bleeding with longer-term thienopyridine use versus a decrease in VLST events.
In conclusion, we have developed a clinical risk score for predicting VLST following DES implantation that identifies populations with a 5-fold variation in the risk for VLST during the second year after DES. Use of this model in clinical practice may enable more selective risk-based strategies (i.e., thienopyridine continuation, testing for antiplatelet therapy responsiveness) in patients at increased risk for developing VLST.
Acknowledgements
The authors would like to thank Kevin Najarian, MS and Hsini (Terry) Liao, PhD, for their contributions in the statistical design and analysis and the late Donald S. Baim, MD for his editorial input.

