The PRAGUE-26 trial (ClinicalTrials.gov: NCT05493163) is a non-industry-sponsored, prospective, multicentre, randomised, active-controlled, unblinded, parallel-group study evaluating clinical outcomes in patients with intermediate-high risk acute pulmonary embolism (PE), comparing catheter-directed thrombolysis (CDT) to standard anticoagulation therapy. Patients with intermediate-high risk acute PE meeting inclusion criteria and not having any exclusion criteria are randomised at a 1:1 ratio to receive either CDT or standard anticoagulation therapy alone. The primary outcome is a clinical composite endpoint of all-cause mortality, PE recurrence, or cardiorespiratory decompensation or collapse, assessed within 7 days of randomisation. The secondary outcomes include bleeding complications, first-line therapy failure, cost-effectiveness analysis, and a broad spectrum of functional and patient-reported outcomes over a 2-year follow-up period. The trial aims to enrol 558 patients. As of 24 November 2024, 258 patients have been randomised. PRAGUE-26 seeks to assess the clinical benefits of simple CDT compared to anticoagulation alone in intermediate-high risk acute PE and aims to contribute to the understanding of this interventional approach.
Over the past decade, percutaneous treatment options for intermediate-high and high-risk acute pulmonary embolism (PE) have rapidly evolved. Since 2014, there has been growing evidence for the efficacy and safety of catheter-based treatments for acute PE, supported by data coming from registries, prospective cohorts, and small randomised controlled trials (RCTs)1. However, no large-scale randomised studies comparing interventional to standard treatment have yet been conducted12.
Currently, two main interventional strategies are under extensive investigation: (a) catheter-directed thrombolysis (CDT), and (b) mechanical thrombectomy. Each approach offers distinct advantages and disadvantages, potentially challenging the current standard of care, particularly in intermediate-high risk PE3. In these patients, CDT has shown some promising results regarding safety and efficacy, regardless of whether simple catheter-directed local thrombolysis or ultrasound-facilitated, catheter-directed thrombolysis (USCDT; EKOS [Boston Scientific]) is used4567. To date, no evidence suggests that one method is more effective than the other89.
The industry-sponsored, randomised HI-PEITHO trial, with an adaptable design allowing for up to 544 participants, is currently underway. This trial is comparing USCDT to anticoagulation alone (the current standard of care) in a preselected group of patients with intermediate-high risk PE and is expected to provide much-needed robust clinical data10. In parallel, the investigator-initiated, non-industry-sponsored, academic RCT, titled “A Multicentre, Randomized Trial of Catheter-directed Thrombolysis in Intermediate-high Risk Acute Pulmonary Embolism (PRAGUE-26)” (ClinicalTrials.gov: NCT05493163), is now in progress. This trial will compare simple CDT (without ultrasound facilitation) to standard anticoagulation alone.
Methods
Study design
PRAGUE-26 is an academic, prospective, multicentre, randomised, active-controlled, unblinded, parallel-group trial designed to evaluate clinical outcomes in patients with intermediate-high risk acute PE, comparing CDT to standard anticoagulation therapy. The study was approved by the Multicentric Ethics Committee at University Hospital Kralovske Vinohrady in Prague and by the official regulatory authority, the State Institute for Drug Control in the Czech Republic.
A flow diagram of the study is shown in Figure 1. The list of PRAGUE-26 investigators and participating institutions is provided in Supplementary Appendix 1. This study adheres to the CONSORT 2010 guidelines for the reporting of randomised controlled trials. A detailed checklist is provided in Supplementary Appendix 2.
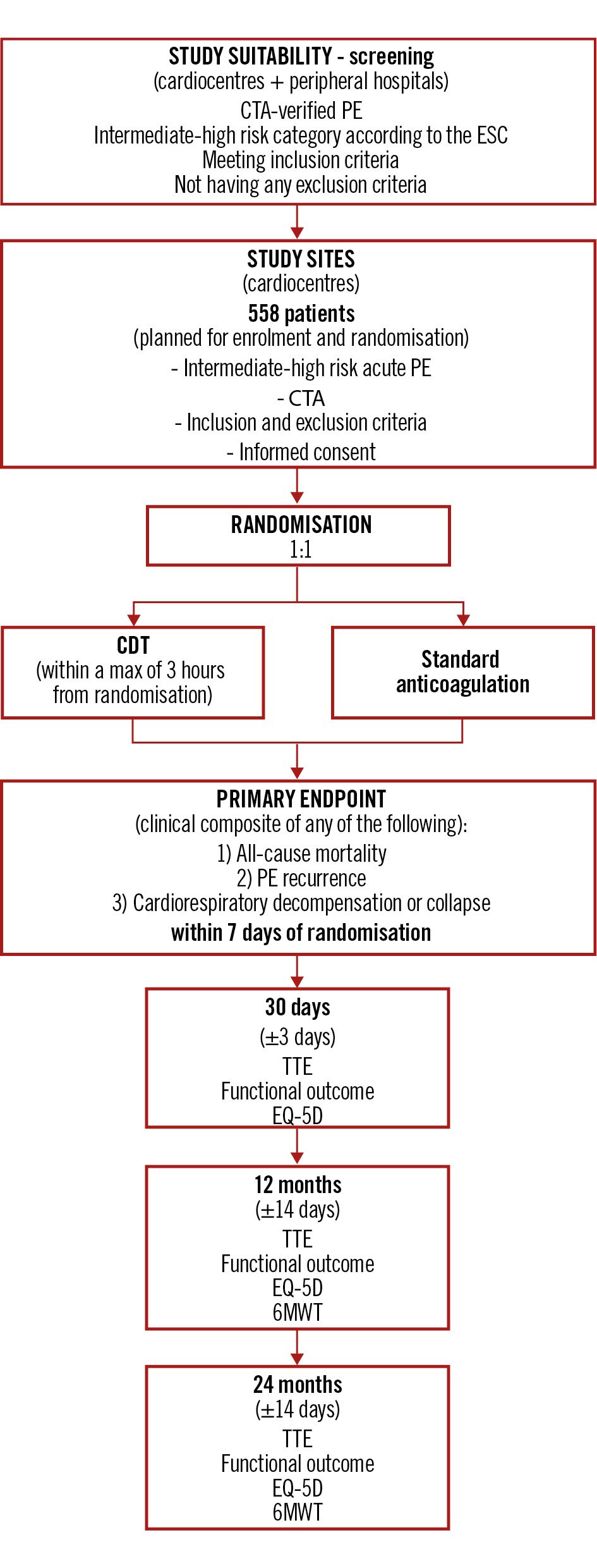
Figure 1. Study flow diagram – PRAGUE-26 CDT: catheter-directed thrombolysis; CTA: computed tomography angiography; EQ-5D: EuroQol 5-Dimension; ESC: European Society of Cardiology; PE: pulmonary embolism; TTE: transthoracic echocardiography; 6MWT: 6-minute walk test
Eligibility criteria
PRAGUE-26 is enrolling intermediate-high risk PE patients as defined in the European Society of Cardiology (ESC) 2019 Guidelines on Acute Pulmonary Embolism, including patients directly admitted to the cardiocentre as well as patients transferred from peripheral hospitals (referral centres); these patients meet the inclusion criteria and do not have any exclusion criteria, as outlined in Table 1.
Table 1. PRAGUE-26: inclusion and exclusion criteria
| Inclusion criteria | 1. Age >18 years and ≤80 years |
| 2. CTA-verified proximal* PE and symptom onset <14 days prior | |
| 3. Intermediate-high risk PE with a sPESI score ≥1 and RV dysfunction# and an elevated biomarker§ (hs-troponin or NT-proBNP) level | |
| 4. Signed informed consent | |
| Exclusion criteria | 1. Active clinically significant bleeding |
| 2. Any haemorrhagic stroke or a recent (<6 months) ischaemic stroke/transient ischaemic attack | |
| 3. Recent (<3 months) cranial trauma or another active intracranial/intraspinal process | |
| 4. Major surgery within 7 days prior | |
| 5. Active malignancy or other severe illness with expected survival <2 years | |
| 6. Haemoglobin level <80 g/L; international normalised ratio >2.0; platelet count ≤100x109; creatinine level >200 µmol/L | |
| 7. Pregnant or breastfeeding, fertility without previous exclusion of gravidity | |
| 8. Allergy to thrombolytics or heparin or low-molecular-weight heparin, contrast allergy, a history of heparin-induced thrombocytopaenia | |
| 9. Floating thrombi in transit through a patent foramen ovale | |
| 10. Participation in another clinical trial | |
| *A perfusion defect in at least one main or one lobar pulmonary artery evident on CTA. #RV/LV ratio ≥0.9 on transthoracic echocardiography or CTA. §hs-troponin I (TnI) >53 ng/L (males) or >34 ng/L (females), hs-troponin T (hs cTnT) >14 ng/l; NT-proBNP level >600 pg/mL, BNP >100 pg/mL. CTA: computed tomography angiography; LV: left ventricular; NT-proBNP: N-terminal pro-brain natriuretic peptide; PE: pulmonary embolism; RV: right ventricular; sPESI: simplified Pulmonary Embolism Severity Index | |
Primary outcome
The primary outcome of the PRAGUE-26 study is a combined clinical endpoint, a composite of any of the following three events within 7 days of randomisation: all-cause mortality, PE recurrence (non-fatal, symptomatic, and objectively confirmed), or cardiorespiratory decompensation or collapse.
Cardiorespiratory decompensation or collapse is defined by at least one of the following criteria: (a) cardiac arrest or the need for cardiopulmonary resuscitation (CPR) at any time between randomisation and day 7; (b) signs of shock, defined as new-onset persistent arterial hypotension (systolic blood pressure below 90 mmHg or systolic blood pressure drop of at least 40 mmHg, sustained for more than 15 minutes despite an adequate volume status, or the need for vasopressors to maintain systolic blood pressure of at least 90 mmHg), accompanied by end-organ hypoperfusion (altered mental status, oliguria/anuria, or increased serum lactate >2 mmol/L) at any time between randomisation and day 7; (c) commencement of extracorporeal membrane oxygenation; (d) intubation or initiation of non-invasive mechanical ventilation at any time between randomisation and day 7; (e) National Early Warning Score (NEWS) of 9 or higher, between 24 hours and 7 days after randomisation, confirmed by consecutive measurements taken twice, 15 minutes apart11.
The NEWS score is an internationally standardised scoring system used in critical care medicine to objectively assess patient deterioration and potential treatment failure.
Secondary outcomes
The secondary outcomes of the trial include all the individual components of the primary endpoint, as well as the following: first-line therapy failure (defined as the administration of systemic thrombolysis during the index hospitalisation); ischaemic or haemorrhagic stroke; all serious adverse events; duration of hospitalisation (intensive care unit/coronary care unit and overall hospital stay); hospitalisation cost (cost-effectiveness analysis); bleeding complications classified by GUSTO as major (moderate and severe) bleeding, by the International Society on Thrombosis and Haemostasis (ISTH) as major bleeding, and all bleeding complications according to the Bleeding Academic Research Consortium (BARC) criteria; echocardiographic measures of right ventricle recovery and pulmonary artery hypertension; functional status assessed by the World Health Organization (WHO) functional class; functional status as measured by the 6-minute walk test (6MWT); quality-of-life assessment using the EuroQol 5-Dimension (EQ-5D) scale; and diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH).
All primary and secondary objectives are summarised in Supplementary Appendix 3, including prespecified time frames for evaluation.
Enrolment
Prerandomisation evaluation
All patients, including those directly admitted to the cardiocentre and those transferred from cooperating peripheral hospitals, will undergo routine clinical evaluation and standard-of-care testing (computed tomography angiography [CTA], transthoracic echocardiography, and laboratory testing) for diagnosis and risk stratification of acute PE. Patients with intermediate-high risk PE who meet the inclusion criteria and do not meet any exclusion criteria will be deemed suitable for study participation. The State Institute for Drug Control (Czech Republic, regulatory authority) insisted on the exclusion of elderly patients (over 80 years of age) due to the perceived higher risk of bleeding from thrombolysis in this population.
There is no time limit from the CTA-confirmed diagnosis of acute PE to randomisation, but investigators are encouraged to take measures to minimise the duration from diagnosis to randomisation.
Randomisation
The randomisation of study participants takes place at study sites (tertiary care cardiocentres) capable of percutaneous interventions for ST-segment elevation myocardial infarction on a 24/7 basis. After providing written informed consent, the participants are randomised via a web-based software, with a 1:1 allocation to either the interventional group (CDT) or the standard care group (anticoagulation alone).
Randomisation into treatment groups is stratified by the following criteria: age, sex, unilateral or bilateral acute PE, time from the diagnosis of acute PE (diagnostic CTA at <24 h or >24 h), and direct admission to the cardiocentre.
Patients randomised to the interventional CDT group should undergo the procedure as soon as possible after randomisation, ideally within a maximum of 3 h, per protocol.
Invasive procedure, anticoagulation management
Following prior exclusion of deep vein thrombosis via ultrasound or CTA, venous access is obtained under ultrasound guidance via the common femoral vein. The choice of a double-lumen 8 Fr introducer (single access site) or two 4 Fr introducers (two ipsilateral access sites) is left to the operator’s discretion. Subsequent procedural steps are detailed in a previously published pilot study4. Operators are strongly advised against the use of hydrophilic wires in the pulmonary arteries because of the risk of vessel perforation. Any 4 Fr valved infusion catheter with an active zone of 10 cm may be used at the operator’s discretion.
After each catheter placement, a bolus of 1 mg alteplase (Actilyse [Boehringer Ingelheim]) per catheter is administered, followed by a continuous infusion at 1 mg/h per catheter for 9 h (total dose of 10 mg for unilateral and 20 mg for bilateral PE).
During CDT, intravenous unfractionated heparin is administered to maintain a target activated partial thromboplastin time (aPTT) of 50-60 s. Following local thrombolysis, the catheters are removed, and anticoagulation with unfractionated heparin (without a bolus) is continued to reach a target aPTT of 70-90 s. The 8 Fr introducer (or two 4 Fr introducers) is removed from the femoral vein 60 min after the end of the alteplase infusion, and the access site is manually compressed for 10 min.
In patients undergoing the CDT procedure, additional laboratory tests (blood count, aPTT, international normalised ratio, fibrinogen) are recommended 6 hours after alteplase infusion initiation.
Anticoagulation
Before randomisation, all patients are treated with intravenous unfractionated heparin (to a target aPTT of 70-90 s) or a full therapeutic dose of subcutaneous low-molecular-weight heparin (LMWH). For patients treated with LMWH who are randomised to CDT, the procedure should be postponed for 8 h after the last LMWH dose. Anticoagulation management during CDT is as described above. Patients in the standard care group continue therapeutic anticoagulation with either unfractionated heparin or LMWH, in accordance with current guidelines. A subsequent switch to oral anticoagulation is at the discretion of the treating physician, but not earlier than 24 hours post-randomisation.
Follow-up evaluation
Study participants will be followed for a period of 2 years after randomisation. The study assessment schedule, along with all subsequent procedures, is summarised in Table 2.
Table 2. Study assessment schedule
| Period | Screening | Randomisation | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 (24±3 hours) | Day 3 (48±6 hours) | Discharge | Day 30 (±3 days) | 12 months (±14 days) | 24 months (±14 days) | ||
| Informed consent | x | |||||||
| Inclusion/exclusion criteria | x | x | ||||||
| Demography/medical history | x | |||||||
| Concomitant medication | x | x | x | x | x | |||
| Height and weighta | x | x | x | |||||
| Vital signsb | x | x | x | x | x | x | x | x |
| Biochemistryc | x | |||||||
| Haematologyc | x | |||||||
| Coagulationc | x | |||||||
| Transthoracic echocardiography | x | x | x | x | x | |||
| CDT procedured | x | |||||||
| WHO Functional Class | x | x | x | x | ||||
| EQ-5D | x | x | x | |||||
| 6MWT | x | x | ||||||
| AE assessmente | x | x | x | x | x | x | ||
| aHeight in cm, weight in kg. bBlood pressure in mmHg, heart rate in beats/min, body temperature in °C, respiratory rate in breaths/min. cLaboratory tests – components of routine clinical care (including hs-troponin and NT-proBNP and pregnancy [serum or urine] testing in women before menopause). dOnly in patients allocated to the CDT group. eAt each study visit, the investigator will determine whether any (serious) adverse events ([S]AE) have occurred. All serious adverse events and adverse events, depending on local regulatory requirements, will be recorded in a timely manner on the electronic case report form by the investigator (or any dedicated site personnel) and will include the event start and stop date, description of event, severity, and relatedness to the index procedure and the study medication. Notification of suspected unexpected serious adverse events will be in accordance with guidelines KLH-21. CDT: catheter-directed thrombolysis; EQ-5D: EuroQol 5-Dimensional; WHO: World Health Organization; 6MWT: 6-minute walk test | ||||||||
Data management
Pseudonymised data will be entered into an electronic case report form provided and managed by the Third Faculty of Medicine, Charles University, Prague. All data and relevant study documentation will be stored at the trial sites for at least 25 years in compliance with Czech Republic law.
Data monitoring
All clinical events (endpoints, adverse events, and others) will be assessed and reviewed by a clinical adjudication event committee.
The data safety monitoring board (DSMB) will oversee the study’s progress and ensure the safety of patients is maintained. The DSMB will review accumulating data on a regular basis, with planned analyses at 25%, 50%, and 75% of study enrolment.
Sample size calculation
Based on three studies – the PEITHO study (with a primary composite endpoint of death or haemodynamic collapse, showing an incidence of 5.6% in the standard arm)12, a study published by Becattini (reporting early mortality of 6.0-7.7%)13 and our randomised pilot study4 – we estimated an incidence of the primary composite endpoint of 1.5% in the CDT group and 6.0% in the standard care group. The power calculation is shown in Table 3.
Table 3. Study parameters, power calculation
| Primary endpoint – composite of any death, PE recurrence, cardiorespiratory decompensation/collapse (time frame: within 7 days of randomisation) | |
|---|---|
| Incidence (in CDT group; N=279) | 1.5% |
| Incidence (in standard anticoagulation group; N=279) | 6% |
| Alpha (N=558) | 0.05 |
| Beta (N=558) | 0.2 |
| Power (N=558) | 0.8 |
| Power calculation – sample size calculator: https://clincalc.com/stats/samplesize.aspx. CDT: catheter-directed thrombolysis; PE: pulmonary embolism | |
Recruitment status
As of 24 November 2024, 258 participants have been enrolled and randomised into the PRAGUE-26 study across 8 active sites in the Czech Republic. Completion of enrolment and reporting of the primary endpoint are anticipated in January 2026. The number of centres may be increased depending on the enrolment rate.
Statistical analysis plan
Data will be analysed on an intention-to-treat basis, with a secondary per-protocol analysis also planned. Interim efficacy and safety analyses are scheduled at 25%, 50%, and 75% of the enrolled patient target, with alpha spending adjusted using the O’Brien-Fleming method to control the overall type I error rate. Based on the review of clinical and safety events, the DSMB will provide recommendations on the study’s continuation or may advise early termination.
Discussion
The interventional treatment of acute PE is rapidly advancing, and there is an urgent need for RCTs that are specifically designed and adequately powered to assess the safety and real clinical benefits of interventional approaches, particularly in intermediate-high risk PE. To this end, several large-scale clinical trials are underway, comparing different interventional methods14 against each other or evaluating various catheter-based interventions relative to the standard of care1015.
To the best of our knowledge, the PRAGUE-26 study is the second largest interventional, multicentre, randomised trial currently being conducted in this field (after PEERLESS II; ClinicalTrials.gov: NCT06055920). Similar to the industry-sponsored HI-PEITHO study (NCT04790370), the academic PRAGUE-26 trial is challenging the current standard of care in intermediate-high risk acute PE by employing a simple, low-cost CDT approach. With its rigorous design and well-defined endpoints, the PRAGUE-26 study is sufficiently powered to determine whether CDT (without ultrasound facilitation) is superior to standard anticoagulation alone in intermediate-high risk patients. In addition, the study is expected to provide valuable information on bleeding complications, patient-oriented functional outcomes, and the cost-effectiveness of the interventional approach.
PRAGUE-26 is enrolling patients who are either directly admitted to tertiary care interventional centres or referred from peripheral hospitals. The impact of routinely transporting patients with intermediate-high risk acute PE for CDT is not yet known, although this strategy may potentially benefit these patients.
The optimal timing of the interventional procedure, thrombolytic dose, and infusion duration remain unclear. In our trial, we chose a total dose of 20 mg of alteplase for bilateral acute PE (10 mg for unilateral PE). Previous studies that have used local thrombolysis have employed similarly low doses, and it is reassuring that the risk of intracranial or life-threatening bleeding does not appear to be increased56716. One small study has investigated dosing and infusion duration for alteplase, with a total dose range of 8 mg to 24 mg; a higher dose appeared to be numerically more effective5. These data provided the scientific rationale for our selected dose of alteplase. Independently, the ongoing multicentre HI-PEITHO study of ultrasound-assisted local thrombolysis has chosen a similar dosing approach, with 9 mg for unilateral and 18 mg for bilateral PE10.
Finally, PRAGUE-26 will assess the feasibility of percutaneous pulmonary interventions (CDT) in cardiac interventional laboratories without prior extensive experience with these procedures (PE-naïve interventional centres). The simplicity and relatively low cost of this intervention might encourage broader adoption among interventional centres worldwide that are not currently offering PE interventions.
Limitations
The PRAGUE-26 study is being conducted at high-volume, primarily tertiary care centres with 24/7 access to cardiac catheterisation, providing the highest level of care available. Although the study enrols intermediate-high risk patients initially admitted and diagnosed in peripheral hospitals, the randomisation process and subsequent care are conducted exclusively at high-volume centres, even for patients in the standard care group. It is important to note that in a real-world setting, only about half of all patients with acute PE are treated at high-volume centres, while the remaining stay at low-volume (peripheral) hospitals17. To demonstrate the superiority of CDT over anticoagulation therapy alone, it is necessary to avoid potential bias arising from differences in care levels between high- and low-volume centres by conducting the trial solely at high-volume centres.
Conclusions
PRAGUE-26 (ClinicalTrials.gov: NCT05493163) aims to evaluate the clinical benefits of simple catheter-directed thrombolysis compared to anticoagulation alone in patients with intermediate-high risk acute PE. The PRAGUE-26 trial will provide insights into whether simple CDT is superior to standard anticoagulation alone in intermediate-high risk acute PE. Regardless of outcome, it will also offer valuable information on the efficacy, safety, and cost-effectiveness of this straightforward interventional approach.
Acknowledgements
We thank all investigators and healthcare professionals involved in the study for their tremendous dedication and support of the PRAGUE-26 study. Author contributions are listed in Supplementary Appendix 4.
Funding
This clinical research is funded by the Ministry of Health of the Czech Republic, grant no. NU23-02-00446 and further supported by the Charles University Czech Republic Research Program COOPERATIO - Cardiovascular Science and CarDia (Programme EXCELES, ID Project No. LX22NPO5104) - funded by the European Union - Next Generation EU.
Conflict of interest statement
J. Kroupa reports receiving consultation fees from Terumo and Boston Scientific; and holds stocks in Medtronic and Inari Medical. V. Kocka reports receiving consultation fees from Medtronic, Abbott, and Philips. M. Poloczek reports receiving consultation fees from Abbott. P. Tousek reports receiving consultation fees from Medtronic. M. Pliva holds stocks in Inari Medical. M. Sluka reports receiving consultation fees from Abbott; and lecturer fees from Edwards Lifesciences. The other authors have no conflicts of interest to declare related to this work.
Supplementary data
To read the full content of this article, please download the PDF.

