![]()
Abstract
Growing populations and ageing demographics lead to an increased burden of ischaemic heart disease and related cardiovascular interventions, resulting in pressure on healthcare systems. Although the healthcare system in Turkey has undergone comprehensive remodelling over the last decade, there are many challenges to overcome, including better reimbursement for cardiovascular interventions, standardisation of interventional cardiology services and research-related activities. In this manuscript, we present an overview of coronary and structural heart interventions in Turkey, as well as providing information on current reimbursement policies and the healthcare system.
Demographics and organisation
Turkey is located at the crossroads between Europe and Asia and had over 78,740,000 inhabitants in December 2015, making it the third most populous country in Europe. Compared to the previous year, Turkey has seen a rise of 1,045,000 in the population.
Interventional procedures are performed in 341 catheterisation laboratories, 142 of which are in private hospitals. Twenty-four percent of catheterisation laboratories are located in the city of Istanbul and 10% in the city of Ankara, with populations of around 15 and 5 million, respectively.
Our interventional working group, the Turkish Society of Cardiology-Association of Percutaneous Cardiovascular Interventions (TSC-APCI) (http://www.tkdgirisimsel.org/en) was founded 25 years ago. The main undertakings of the association include organising training activities in a wide range of cardiovascular interventions in order to help increase the level of knowledge and skill set of the members. This will not only standardise cardiovascular interventions but also raise the quality of their practice. This is extremely important, especially since cardiology subspecialities are not officially recognised in Turkey, leaving a combination of local facilities, demands and personal interests to drive one’s ability/decision to practise as an interventionalist and forms part of the reason for the continuous educational activities organised by the TSC-APCI. Finally, although work is ongoing to create one, currently there is no structured national registry for percutaneous interventions in Turkey. The data below were extracted as being simply the total number of procedures from the National Insurance Institution.
The healthcare sector in Turkey has undergone comprehensive remodelling in the last decade and recent investments have raised the quality of the country’s overall healthcare service. Such advancements have encouraged the use of technology and have helped expand the availability of procedures all over the country. In addition, the country’s social security system has seen a major transformation, which has greatly improved its efficiency. The evolution of healthcare was constructed around the basis of unifying the control of different social security funds into a single institution. Under the “Universal Health Insurance” system, all residents registered with the Social Security Institution can now receive medical treatment, free of charge, in any Ministry of Health (MoH) or university hospital. The service also applies to most private hospitals, whereby residents can receive medical services with considerably discounted additional surcharges. Access to these services in private institutions is crucial: whilst MoH hospitals represent the larger proportion of the medical system, over the last decade Turkey has seen a significant rise in the number of private hospitals that have increasingly helped to alleviate the growing demands on the healthcare service. The state also pays health premiums for the portion of the population classified as “low income” holding a green card. Furthermore, Universal Health Insurance now covers all residents under 25 years of age and has also been expanded to stateless persons and refugees. Such amendments have resulted in a more expansive healthcare system which has allowed the delivery of services in a fair and justifiable manner.
Data from 2015 reveal that 40.3% of the total number of deaths in Turkey were attributed to circulatory diseases, making them the leading cause of mortality. Ischaemic heart disease represented 40.5% of these deaths related to circulatory disease. Furthermore, between 2012 and 2030, the number of deaths from cardiovascular disease is expected to rise at a rate of 27.8% per year. Such a progression in diseases poses a great financial burden that leads to strict reimbursement policies for the interventions.
Coronary interventions
Naturally, these policies differ between coronary and structural diseases. Coronary cases are usually reimbursed as packages. For example, the hospital is paid around 150 US dollars for diagnostic coronary angiography, regardless of whether it is a private or government-run hospital. A PCI case is roughly 400 US dollars, while balloons are refunded separately. Although stents are also reimbursed separately, drug-eluting stents are only reimbursed if their size is 3.0 mm or under. Bioresorbable scaffolds are not reimbursed. At present, the amount paid for intravascular ultrasound catheters is around 60% of their sale price, leading to considerable underutilisation of the technology. Similarly, fractional flow reserve (FFR) guidewires are also underutilised because they were not completely reimbursed until recently. An additional obstacle interventionalists face is the limited reimbursement of adjunctive devices for complex procedures, such as microcatheters that are used for chronic total occlusions. Even the most complicated procedures are subject to packages, resulting in reluctance on the part of many interventionalists to perform these procedures, as most of the equipment easily exceeds the reimbursed amount. Table 1 shows some of the numbers of procedures over recent years. Peripheral interventions, including carotid stenting, are also increasingly being performed by the cardiologists. These procedures are subject to more flexible reimbursement policies.
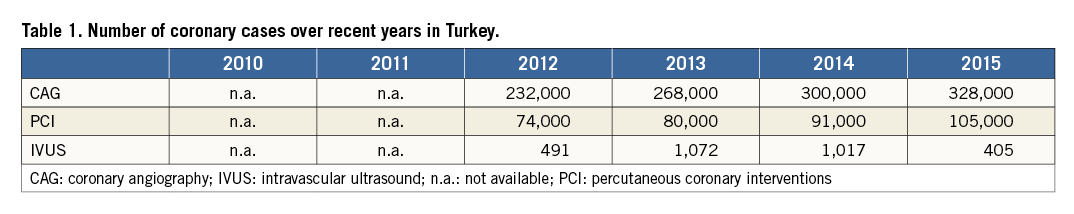
In Turkey, each year, in excess of 100,000 patients are admitted with an ST-segment elevated myocardial infarction. Prior to the onset of the Stent for Life (SFL) project, reperfusion was mainly achieved via thrombolysis and in 2010 only 8% of patients with acute STEMI had access to primary PCI1. As of 2009, Turkey joined the SFL project and, at the start, 18 pilot cities were included around the country. The positive effects of this project can be seen in the growing number of primary PCIs: the cities with pilot studies running have been able to achieve a 90% rate of PCI, as well as a mean door-to-balloon time of 54±43 minutes. Although the number of cities participating in the SFL project is increasing, there are various challenges that are yet to be overcome, including the shortage of staff.
Structural heart interventions
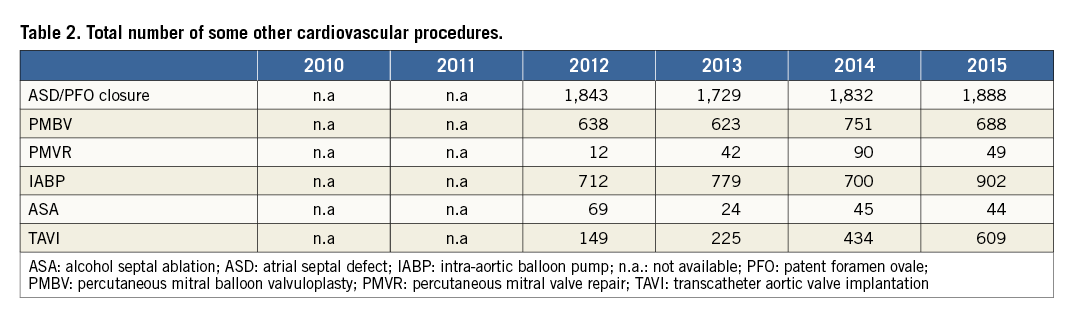
Percutaneous treatment of congenital diseases, including patent foramen ovale, are reimbursed completely in the form of packages, with any devices used reimbursed separately. On the other hand, transcatheter aortic valve implantation (TAVI) procedures are only reimbursed in teaching or step 3 university hospitals, for a selected group of high-risk patients. In Turkey, TAVI procedures cost five times more than aortic valve surgery, which is the main reason for the restricted use of this technology. Presently, percutaneous mitral valve repair procedures are not reimbursed (Table 2). Reimbursement for left atrial appendix closure and annuloplasty devices is pending: these are expected to receive reimbursement soon. Therefore, currently, a small number of these latter procedures are performed based on private insurance or out-of-pocket payments.
Conclusion
Despite all the recent changes in healthcare and interventional cardiology in Turkey, many challenges have yet to be overcome. First, further developments in reimbursement policies are essential in order to keep up with innovations in interventional cardiology. As an association, we should encourage and negotiate with the MoH for these improvements. Secondly, increasing the research activities in which TSC-APCI is involved is an ongoing goal for the team. This involves building national registries, initiating or sponsoring multicentre trials, as well as participating in multinational registries or studies.
Conflict of interest statement
The authors have no conflicts of interest to declare.

