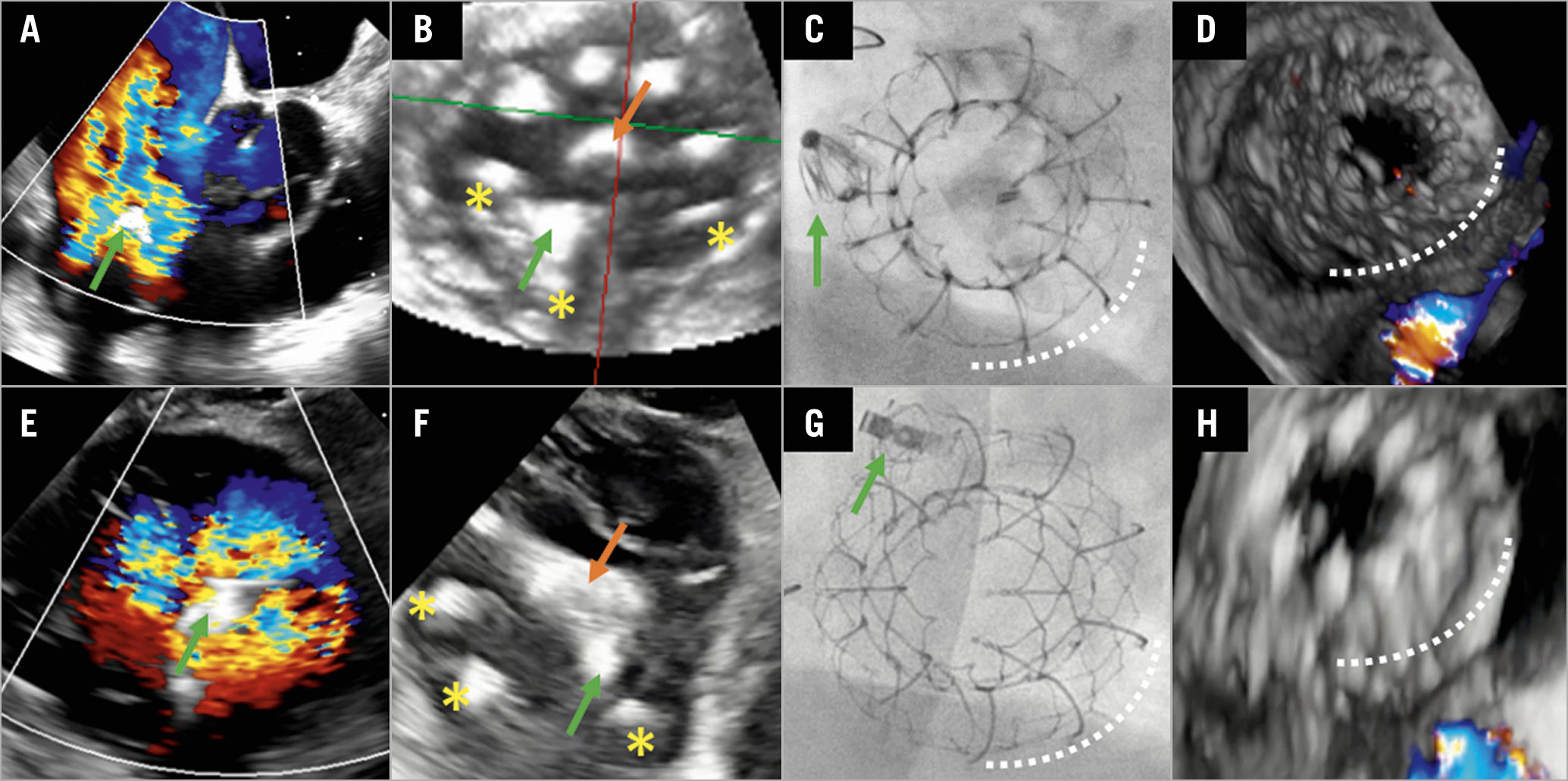
Figure 1. Transfemoral tricuspid valve replacement after failed leaflet repair in two patients. A) First patient. Transoesophageal echocardiography (TEE; right ventricular [RV] inflow/outflow view) showing tricuspid single leaflet device attachment (SLDA) on the posterior leaflet of the PASCAL device (green arrow) leading to torrential tricuspid regurgitation (TR). B) TEE (transgastric en face view) during EVOQUE implantation (orange arrow marking the delivery catheter) showing the anchors (yellow asterisks) capturing the PASCAL device (green arrow). C) Fluoroscopy at the end of the procedure with the PASCAL (green arrow) and EVOQUE (white dotted line) (left anterior oblique [LAO] 40°/caudal 15°). D) Final result (TEE, three-dimensional [3D] with colour Doppler in systole) after EVOQUE (white dotted line) implantation with trace residual TR. E) Second patient. TEE (transgastric en face view) with torrential TR after SLDA of the PASCAL device (green arrow) on the septal leaflet. F) – H) Corresponding images to panels B-D for the second patient.
An 84-year-old high-risk female patient (EuroSCORE II 31.9%, STS-PROM 11.3%) was referred for treatment of symptomatic severe tricuspid regurgitation (TR 3+). Left ventricular function was preserved in the absence of pulmonary hypertension. She underwent successful transcatheter leaflet repair with posteroseptal placement of one PASCAL device (Edwards Lifesciences, Irvine, CA, USA), resulting in good TR reduction (TR 1+). Follow-up echocardiography one month after the procedure revealed a single leaflet device attachment (SLDA) on the posterior leaflet (Figure 1A, Moving image 1). Detailed analysis by transoesophageal echocardiography (TEE) demonstrated no option for further clip-based repair due to laceration of the septal leaflet and a large coaptation gap of 10 mm.
Due to persisting symptoms (New York Heart Association [NYHA] Class IV), prominent peripheral oedema, and new torrential TR (5+), she was evaluated for treatment by valve replacement. After confirmation of eligibility (suitable annular dimensions and adequate TEE imaging, taking into account possible shadowing by the PASCAL device) within a compassionate use programme and approval of the national authorities, the patient underwent successful transcatheter tricuspid valve replacement using an EVOQUE 52 mm valve (Edwards Lifesciences). The EVOQUE consists of a self-expanding nitinol frame with a bovine pericardial trileaflet valve and circumferential anchors using the valve apparatus for stabilisation1. The bioprosthesis was positioned with two anchors flanking and stabilising the PASCAL device to ensure optimal alignment of the flexible outer frame with the tricuspid annulus (Figure 1B, Figure 1C, Moving image 2, Moving image 3). Despite the technical challenge, the procedure time of 113 minutes was comparable to our other early EVOQUE experience. Post-procedural echocardiography showed residual trace TR, no paravalvular leakage (Figure 1D, Moving image 4) and a mean transprosthetic gradient of 3 mmHg. At four-month follow-up, the patient reported significant symptom improvement (NYHA Class II), resolution of peripheral oedema, and a weight loss of 15 kg. Six-minute walking distance improved by 190 metres and echocardiography confirmed good positioning of the prosthesis without valvular or paravalvular residual TR, and stable gradient.
In a similar setting, an 85-year-old male patient (STS-PROM 9.5%; baseline TR 3+, TR after SLDA 5+ with evidence of leaflet tear) with SLDA of one PASCAL device on the septal leaflet (Figure 1E, Moving image 5) was successfully treated with the implantation of an EVOQUE 52 mm valve, procedure time 75 minutes (Figure 1F-Figure 1H, Moving image 6-Moving image 8). In both cases, previous antithrombotic treatment with novel oral anticoagulants (NOACs) was continued.
After failed leaflet repair of TR patients are often left untreated due to technical and/or anatomical limitations. Our cases illustrate the potential of novel transcatheter tricuspid valve replacement therapies and encourage thinking outside the box.
Conflict of interest statement
J. Hausleiter declares support for research projects paid to the institution from Abbott Vascular and Edwards Lifesciences, and has received speaker honoraria from Abbott Vascular and Edwards Lifesciences. M. Näbauer has received speaker honoraria from Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Transoesophageal echocardiography (TEE; RV inflow/outflow view) of the first patient showing tricuspid single leaflet device attachment (SLDA) on the posterior leaflet of the PASCAL device.
Moving image 2. TEE (transgastric en face view) of the first patient during EVOQUE implantation showing the anchors capturing the PASCAL device.
Moving image 3. Fluoroscopy of the first patient at the end of the procedure with the PASCAL and EVOQUE.
Moving image 4. Final result (TEE, 3D with colour Doppler in systole) of the first patient after EVOQUE implantation.
Moving image 5. TEE (transgastric en face view) of the second patient with torrential TR after SLDA of the PASCAL device on the septal leaflet.
Moving image 6. TEE (transgastric en face view) of the second patient during EVOQUE implantation showing the deployment of the anterior anchors to capture the PASCAL device.
Moving image 7. Fluoroscopy of the second patient at the end of the procedure.
Moving image 8. Final result of the second patient after EVOQUE implantation.

