
Abstract
Aims: No randomised study comparing the outcomes of transcarotid (TC) and transaxillary (TAx) TAVR has been conducted to date. The purpose of this study was to understand which approach should be the preferred alternative by comparing their outcomes using a propensity-matched comparison in a French multicentre registry.
Methods and results: From 2010 to 2018, a French multicentre prospective registry included 502 patients, with 374 undergoing TC-TAVR and 128 TAx-TAVR for symptomatic aortic stenosis. Patients treated through TAx access were matched 1:2 with patients treated through the TC route by using a propensity score (20 clinical, anatomical and procedural variables) and by date of the procedure. The first outcome was mortality at one-month follow-up. The second outcome was one-month stroke/transient ischaemic attack (TIA). In propensity-matched analyses, the incidence of the primary outcome was similar in the TAx and TC groups (TAx 5.5% vs TC 4.5%, OR 1.23, 95% CI: 0.40-3.70). The secondary outcome was similar in TAx (3.2%) and TC (6.8%, OR 0.52, 95% CI: 0.14-1.84). Minor bleeding (2.7% vs 9.3%, OR 0.26, 95% CI: 0.07-0.92) and main access haematoma (3.6% vs 10.3%, OR 0.034, 95% CI: 0.09-0.92) were significantly more frequent with the TC access. One-month clinical efficacy and safety and one-year mortality did not differ according to the different routes.
Conclusions: One-month mortality, one-month stroke/TIA and one-year mortality are similar with TAx-TAVR and TC-TAVR. However, TC-TAVR is accompanied by more minor bleeding and main access haematoma compared with the transaxillary route.
Introduction
The transfemoral (TF) access is the primary access route for the vast majority of patients undergoing transcatheter aortic valve replacement (TAVR) thanks to the refinement of the procedure and material1. In 2019, the penetration of the transfemoral approach was as high as 85% in the USA2 and France3, and 99% in low-risk patients4.
The optimal access route for patients undergoing TAVR who are not candidates for a TF approach has not been clearly elucidated5,6. The use of the transapical and transaortic techniques has been surpassed by other routes7. Possible extrathoracic alternatives for patients not amenable to TF-TAVR have been developed recently, including the transcarotid (TC) and transaxillary/subclavian (TAx) routes.
Recent studies on TAx access have reported similar outcomes, including life-threatening bleeding rates (12%) and 30-day mortality rates (6%), compared to TF procedures8,9. The TAx pathway also has lower 30-day mortality and shorter lengths of hospital stay than transthoracic routes including transapical and transaortic access10,11,12. However, some authors suggest that the TAx route may have a higher stroke rate11,13 and more vascular complications13 than TF access. We previously investigated the safety and feasibility of transcarotid TAVR using self-expanding and balloon-expandable prostheses, managed under general anaesthesia or a minimally invasive strategy with favourable clinical outcomes14,15,16,17. We currently use TC or TAx access as the first-line alternative approach for TAVR whenever the TF access is precluded.
The purpose of this study was to understand which approach should be the preferred alternative by comparing their outcomes using a propensity-matched comparison in a French multicentre registry.
Methods
PATIENT SELECTION
Between 2010 and 2018, consecutive patients undergoing TC and TAx TAVR at four French institutions (Institut Coeur-Poumons, CHU Lille, Lille, France; Hôpital Marie Lannelongue, Le Plessis-Robinson, France; CHU Montpellier, Montpellier, France; CH Lens, Lens, France) were included in a collaborative prospective registry. In all cases, the TF approach was precluded by diseased descending aorta (aortic dissection, aortic aneurysm, porcelain aorta), severe peripheral arterial disease (small calibre ≤5.5 mm, severely tortuous, heavily calcified, dissected, significant stenosis), or prior iliofemoral intervention or surgery. Selection of alternative access was then individualised to each patient’s anatomic features and comorbidities.
The yearly number and proportions of the total number of TAVR cases that were not TF in the four participating centres (2010-2018) are shown in Figure 1, representing around 10% of the total number of TAVR procedures in these institutions. The mean case volume per site per year was 4.0 for TAx and 11.6 for TC procedures.
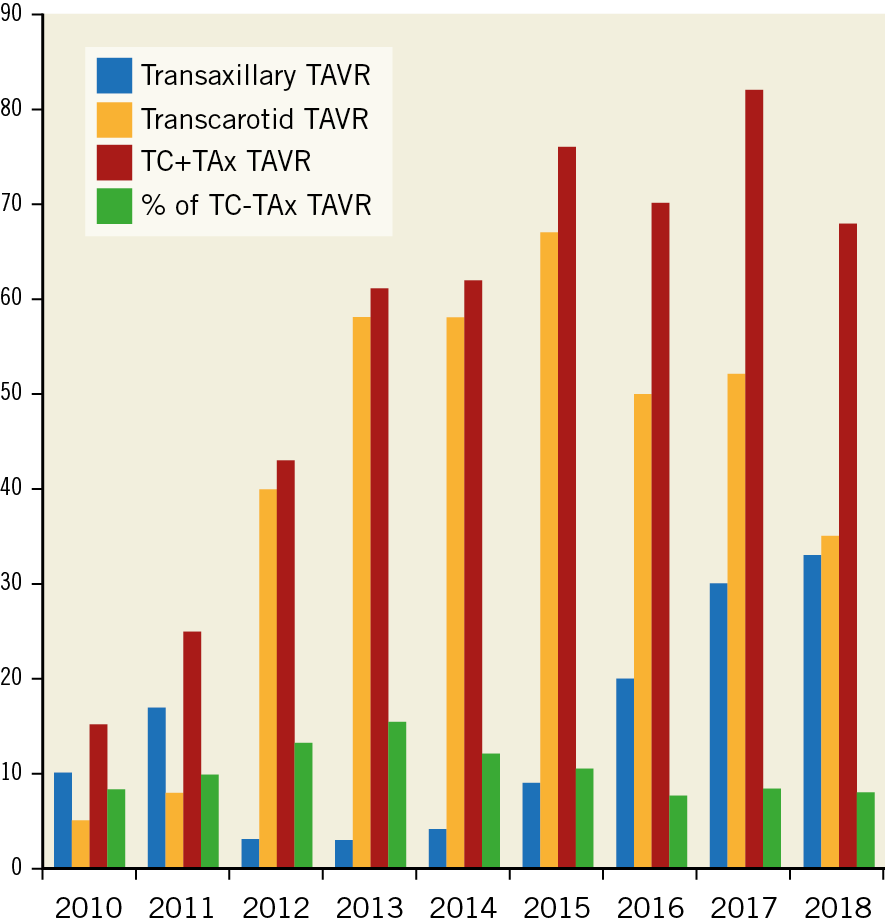
Figure 1. Yearly number and proportion of total transcarotid (TC) and transaxillary/subclavian (TAx) TAVR procedures.
Details concerning preprocedural screening and procedural technique can be found in Supplementary Appendix 1 and Supplementary Appendix 2.
CLINICAL ENDPOINTS
The primary endpoint of interest was one-month mortality18 in TC-TAVR and TAx-TAVR.
Secondary endpoints were one-month stroke/transient ischaemic attack (TIA), one-month clinical efficacy (all-cause mortality, disabling or non-disabling stroke, or hospitalisations for valve-related symptoms or worsening congestive heart failure [CHF]), one-month early safety (all-cause mortality, stroke [disabling and non-disabling], life-threatening bleeding, acute kidney injury [AKI], coronary artery obstruction requiring intervention, major vascular complications, valve-related dysfunction requiring repeat procedure), and one-year mortality. Further analyses involved major outcomes including AKI, aortic regurgitation, bleeding or vascular access complications according to the Valve Academic Research Consortium-2 (VARC-2) consensus definitions18.
All medical files were carefully reviewed and, in case of doubt, clinical events were adjudicated by a medical committee of two physicians.
STATISTICAL ANALYSIS
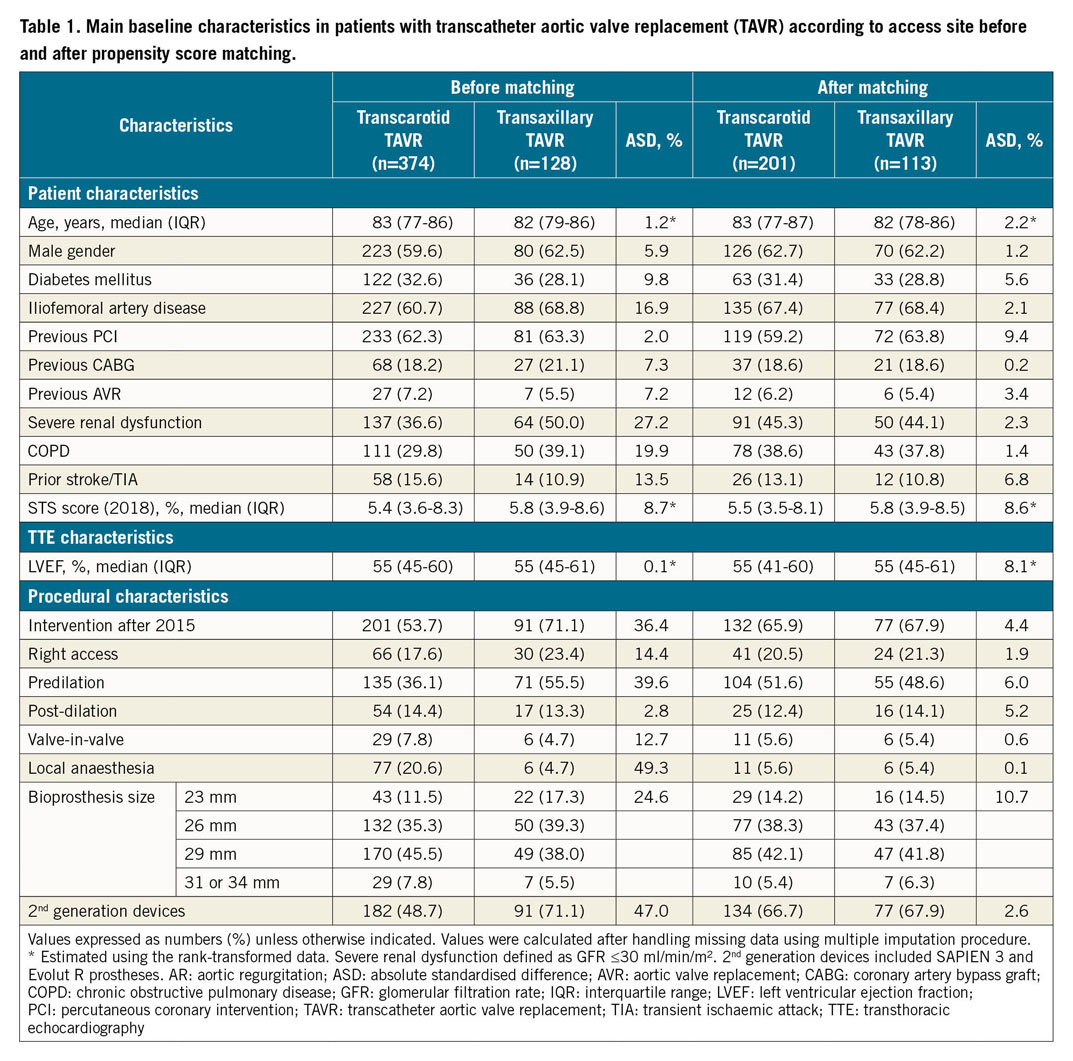
Quantitative variables are expressed as means (standard deviation) in the case of normal distribution or medians (interquartile range) otherwise. Categorical variables are expressed as numbers (percentages). Normality of distribution was assessed using histograms and the Shapiro-Wilk test. Patients were divided into two groups according to the TAVR access site (TAx vs TC). Baseline characteristics were described for the two study groups and the magnitude of the between-group differences was assessed by calculating the absolute standardised difference; an absolute standardised difference >10% was interpreted as a meaningful difference.
Details of the propensity-matched comparison can be found in Supplementary Appendix 3,19.
Statistical testing was conducted at the two-tailed α level of 0.05. Data were analysed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
POPULATION
The number of TC and TAx procedures are shown in Figure 1, and the study flow chart is shown in Figure 2.
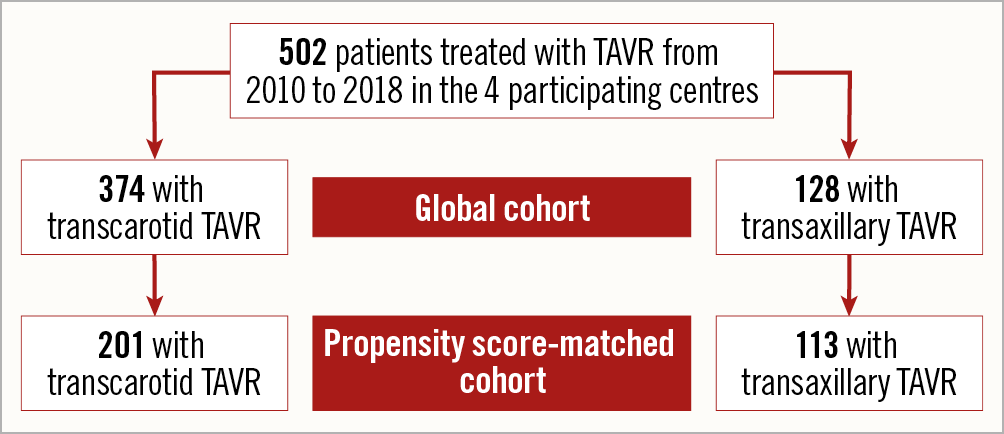
Figure 2. Study flow chart.
Baseline characteristics before matching and handling missing values are presented in Supplementary Table 1. Baseline characteristics according to access, before and after propensity score matching and after handling missing values by multiple imputation are presented in Table 1. The distributions of propensity score according to access are reported in Supplementary Figure 1. Absolute standardised differences between TAVR routes before and after propensity score matching are reported in Supplementary Figure 2.
Before matching, most characteristics were already well balanced (absolute standardised difference ≤10%), except that patients treated with a TAx-TAVR had a higher prevalence of severe iliofemoral disease, severe renal dysfunction, and chronic obstructive pulmonary disease (COPD). TAx-TAVR procedures were more often performed after 2015, with a second-generation prosthesis (SAPIEN 3 [S3; Edwards Lifesciences, Irvine, CA, USA] and Evolut™ R [Medtronic, Minneapolis, MN, USA]), with more predilation and using the right access more frequently. These differences were controlled after propensity score matching (Table 1, Supplementary Figure 2) where 113 TAx-TAVR cases could be matched with 201 TC-TAVR cases.
IMPACT OF ACCESS SITE ON THE OUTCOMES
(i) MORTALITY AND COMPOSITE OUTCOMES
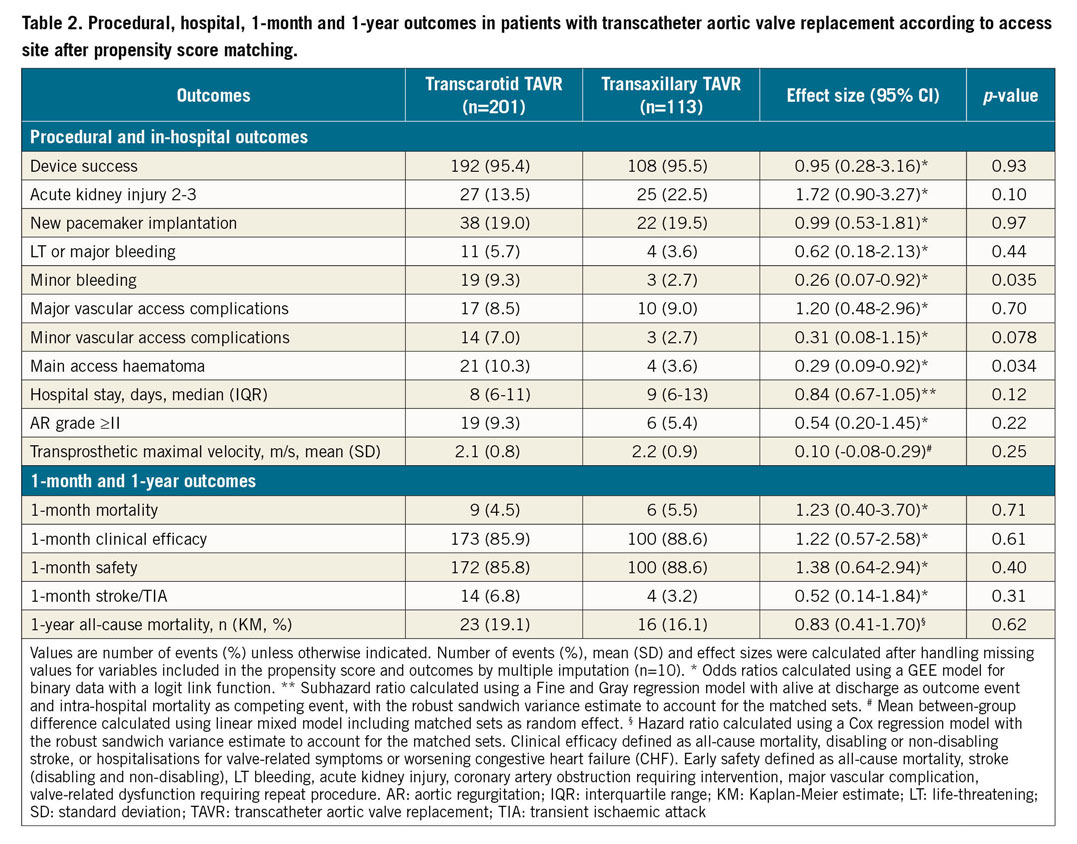
In the propensity score-matched cohort, there was no difference in one-month mortality (TAx 5.5% vs TC 4.5%, odds ratio [OR] 1.23, 95% confidence interval [CI]: 0.40-3.70), one-month early safety (TAx 88.6% vs TC 85.8%, OR 1.38, 95% CI: 0.64-2.94) or clinical efficacy (TAx 88.6% vs TC 85.9%, OR 1.22, 95% CI: 0.57-2.58) between the two access sites (Table 2). There was also no difference in mortality at one-year follow-up, with a matched hazard ratio (HR) of 0.83 (95% CI: 0.41-1.70).
(ii) MORBIDITY INCLUDING VASCULAR COMPLICATIONS
Among procedural and in-hospital events, major vascular access complications, and life-threatening and major bleeding occurred at the same rate in the two groups. Lower rates of minor bleeding (TAx 2.7% vs TC 9.3%, OR 0.26, 95% CI: 0.07-0.92) and main access haematoma (TAx 3.6% vs TC 10.3%, OR 0.29, 95% CI: 0.09-0.92; p=0.034) were also found in patients treated through the TAx route, while the difference in minor vascular access complications did not reach the significance level (TC 7.0% vs TAx 2.7%, OR 0.31, 95% CI: 0.08-1.15; p=0.078). There was no significant difference in post-procedural mean transprosthetic maximal velocity between the two groups (mean difference between TAx and TC=0.10; 95% CI: −0.08-0.29).
(iii) CEREBROVASCULAR EVENTS
Overall, there were 30 (6.0%) VARC-2-defined one-month cerebrovascular events (16 [3.2%] strokes [NIHSS score 1 n=1, 2 n=1, 3 n=3, 4 n=3, 5 n=3, 6 n=4, and 8 n=1] and 14 [2.8%] TIAs), as assessed by clinical and neuroimaging criteria. Nine patients with stroke (56%) had no sequelae at hospital discharge. Cerebrovascular events happened shortly after the procedure (n=15 at day 0, n=5 at day 1, n=2 at day 2, n=3 at day 3, n=2 at day 5, n=2 at day 6, n=1 at day 11). The clinical deficits were localised as ipsilateral (n=8) or contralateral (n=12) to the vascular access site. Clinical features of the events included confusion (n=4), hemiparesis (n=4), hemiplegia (n=15) and aphasia (n=5), and Claude Bernard-Horner signs (n=2); neuroimaging showed new ischaemic lesions in the 16 stroke cases (multiple embolic lesions). One patient (0.8%) in the TAx group had a brachial plexus sideration. After propensity matching, the one-month stroke/TIA rate did not differ between the two groups (TAx 3.2% vs TC 6.8%, OR 0.52, 95% CI: 0.14-1.84).
Discussion
Here we describe one of the largest series of patients undergoing TC or TAx vascular access for TAVR. The main findings from this propensity-matched cohort are: (i) TC-TAVR and TAx-TAVR have similar one-month mortality, one-month stroke/TIA, and clinical safety and efficacy with balloon-expandable or self-expanding valves, and (ii) less minor bleeding and main access haematoma occur in the TAx access group.
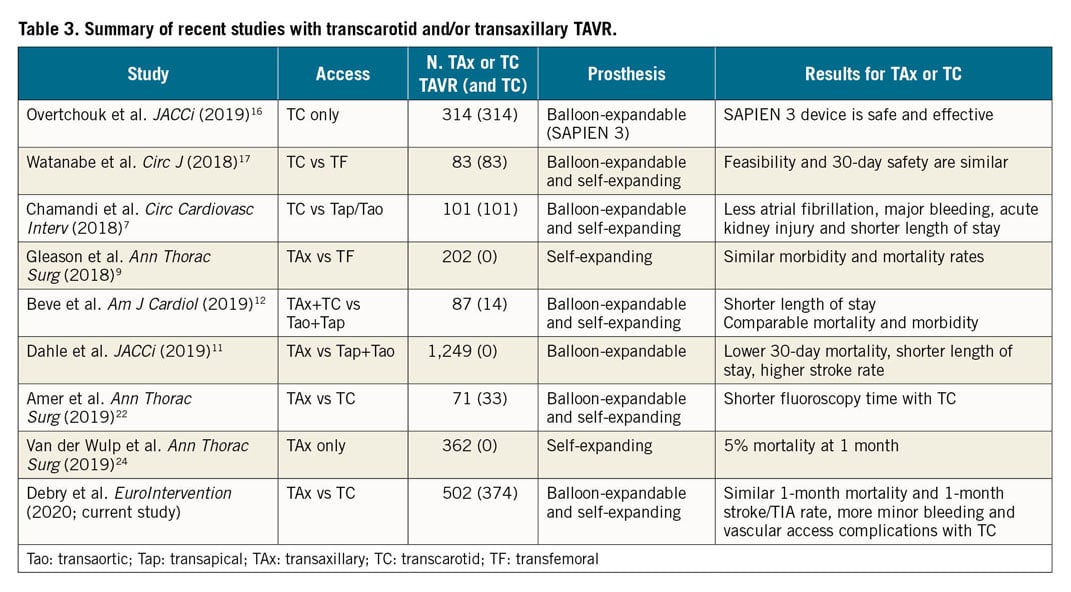
Comparative data about these two most currently used alternative routes for TAVR are unavailable. Numerous articles have previously described these routes in comparison to transthoracic TAVR with favourable results (Table 3).
NON-FEMORAL PERIPHERAL TAVR
A recent French registry in a non-femoral peripheral TAVR cohort found a similar stroke rate (3.35%), lower major vascular complications (0.68%) and higher major bleeding (8.56%)20 compared to our analysis. We also report device success (95.4%) and one-month mortality (5.0%) rates similar to those reported by Dahle et al11. However, this STS/ACC registry11 using only balloon-expandable valves reported extremely low major vascular complications (1.1%) and life-threatening bleeding rates (0.1%). Evaluation of VARC-2 outcomes was not clinically adjudicated in these registries11,16,20. Also, it cannot be excluded that some clinical events, in particular TIA, might have been partly underreported, since they are also significantly lower than reported in clinical trials21. We report similar rates of major vascular complications (9.0% vs 11.9%) and less major and life-threatening bleeding (3.6% vs 11.4%) compared to previous TAx studies9,13 with only self-expanding valves. Potential explanations for this detrimental result with non-femoral TAVR include a procedural learning curve. The TAx or TC access may also be more delicate than iliofemoral ones and prone to vessel dissection, stenosis or thrombosis, and is not accessible for effective manual compression.
COMPARISON OF TAx-TAVR AND TC-TAVR
In line with a previous report22, our study suggests that both approaches are equally safe without difference in early (30-day) and late (one-year) mortality and with similar early stroke/TIA rates. In the case of TC-TAVR, even if it offers a more direct (straighter) access, a less angular path to reach the aortic valve, with less vascular interaction, local complications including local haematoma and minor bleeding, debris embolisation, and the transient reduction in cerebral blood flow during the procedure may explain the non-significant increase in the one-month stroke/TIA rate (6.8% vs 3.2%). The TIA/stroke rate with the TC route did not decrease significantly between the early period (2010-2015 and the use of first-generation prostheses) and after 2015 (7.0% vs 5.4%, p=0.53). The rate of 30-day stroke/TIA that we report is in the upper margin of those previously reported by Amer et al22 (3%), and Mylotte et al15 (3.2%). These figures are also consistent with those of a previous report of ours in which a 5.7% rate of periprocedural cerebrovascular events and an 11.4% rate of global vascular access complications with TC-TAVR were reported14. Passive antegrade carotid perfusion with a temporary femoro-carotid shunt during TC-TAVR was used for the first TC-TAVR patients during the early days (2010) and is no longer used.
The more direct access from the carotid artery allows more precise positioning of the prosthesis, especially for self-expanding valves, which could have resulted in a reduction in periprosthetic regurgitation. However, the angle of the delivery catheter from the carotid artery with respect to the plane of the ring is less favourable than for the left axillary artery which allows the delivery catheter to position itself on the lateral wall of the aorta and finally to cross the plane of the ring more perpendicularly than from the carotid artery (Figure 3).
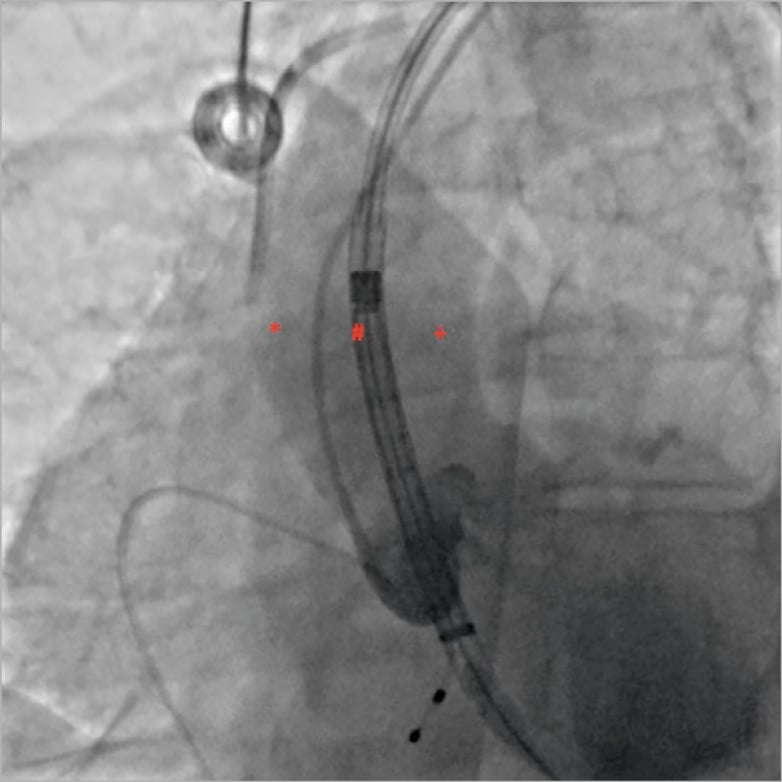
Figure 3. Optimising the delivery of self-expanding valves according to vascular access. When using the left axillary artery, as the delivery catheter positions itself on the lateral wall of the aorta (*), it is better to open the prosthesis at 6 mm under the annulus. When using the carotid or the right axillary artery (+), delivery should start from 0 mm. When arriving at the centre of the native valve (#), and therefore perpendicular to the annulus, delivery should start 3 mm under the annulus.
We also prefer the left axillary/carotid artery over the right because it avoids injury or embolisation to the innominate artery that supplies the right carotid and vertebral distribution. Also, isolated injury to the left axillary/carotid artery is easier to repair than innominate artery injury. While our rate of percutaneous TAx access is low, it remains unclear how surgical and percutaneous TAx approaches may ultimately differ as centres gain more experience with each approach23.
The observed incidence of combined vascular and bleeding complications in both groups underscores the need for a detailed multidisciplinary preprocedural assessment of vascular anatomy to determine the optimal alternative TAVR approach.
Limitations
Limitations to this study are inherent to its non-randomised design. The present findings are derived from observational analyses which are subject to well-known limitations. The first is the potential for confounding by measured or unmeasured variables, which cannot be ruled out, even after propensity score matching or adjustment. A second limitation is missing data in some covariates, including in the propensity score calculation, as well as in outcomes. Although we used multiple imputations to handle missing data as appropriate, we cannot exclude that missing data could have introduced a bias in estimates.
Conclusions
TC-TAVR and TAx-TAVR have similar one-month mortality, one-month stroke/TIA and clinical safety and efficacy with balloon-expandable or self-expanding valves. Less minor bleeding and main access haematoma occurred in the TAx access group. Randomised studies are required to ascertain whether TC-TAVR and TAx-TAVR yield similar results.
|
Impact on daily practice Early post-procedural complications following alternative peripheral accesses for TAVR have dropped significantly over the years with growing experience, and are overall similar through either the TAx or TC route. However, our data may suggest that less minor bleeding and main access haematoma occur in the TAx access group. |
Appendix. Study collaborators
Antoine Bical, MD; Alessandro Cosenza, MD; Augustin Coisne, MD, PhD; Sina Porouchani, MD; Arnaud Sudre, MD; Agnès Mugnier, MD; Natacha Rousse, MD; Emmanuel Robin, MD; Heart Team, Institut Cœur-Poumons, CHU Lille, Lille, France. Tom Denimal, MD; Thibault Pamart, MD; Hugues Spillemaeker, MD; Basile Verdier, MD; Valentin Loobuyck, MD; André Vincentelli, MD, PhD; Mouhamed Djahoum Moussa, MD; Heart Team, Institut Cœur-Poumons, CHU Lille, Lille, France; National Institute of Health and Medical Research U1011, Lille, France. Jean-Christophe Macia, MD; Myriam Akodad, MD; Heart Team, CHU Montpellier, Montpellier, France. Olivier Fabre, MD; Antoine Gommeaux, MD; Heart Team, CH Lens, Lens, France. Laurent Schmutz, MD; Heart Team, CHU Nîmes, Nîmes, France. Alexandre Azmoun, MD; Heart Team, Hôpital Marie Lannelongue, Le Plessis Robinson, France.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

