
Abstract
Aims: Despite advances in understanding the physiological role of collaterals in coronary chronic total occlusions (CTOs), collateral anatomy remains poorly defined. Our aim was to define the anatomy and interventional utility of collaterals within a large population of patients with CTOs.
Methods and results: We studied the coronary angiograms of 481 patients with 519 CTOs at six centres in the UK over four years. Detailed angiographic analysis was performed by interventional cardiologists specialising in CTO percutaneous coronary intervention (PCI). All visible collaterals with a collateral connection (CC) grade ≥1 were recorded. A subgroup of CTOs (n=277) was assessed for interventional capability, defined as whether the collateral supply was able to facilitate retrograde access. We described 45 different collateral patterns: 20 in right coronary artery (RCA), 13 in left anterior descending (LAD), and 12 in circumflex artery CTOs. Septal collaterals from the LAD to the right posterior descending artery (RPDA), and from the posterior descending artery to the LAD were most common, and most often considered as having “interventional capability”.
Conclusions: This is the largest analysis of collateral circulation anatomy in a population of patients with CTOs. We anticipate that these data will be of significant benefit in angiographic analysis and procedure planning for CTO PCI.
Abbreviations
CABG: coronary artery bypass graft
CB: conus branch
CC: collateral connection
CTO: chronic total occlusion
Cx: circumflex
LAD: left anterior descending
OM: obtuse marginal
PCI: percutaneous coronary intervention
PDA: posterior descending artery
PLV: posterolateral ventricular
RA: right atrial
RCA: right coronary artery
RV: right ventricular
SN: sinus node
TIMI: Thrombolysis In Myocardial Infarction
Introduction
With advances in percutaneous treatment of chronic total occlusions (CTOs), the role and interventional utility of the collateral blood supply is of increasing interest. Recent studies have improved our understanding of the role of collaterals in maintaining myocardial viability, and well-developed collaterals in CTOs have been shown to improve cellular function and global myocardial performance1-4. In contrast to the body of work assessing the physiological role of collaterals, collateral anatomy has not been the focus of attention for many years. Studies date back more than 40 years when Levin first attempted to relate the anatomic distribution of collaterals with collateral function. However, there has been no systematic characterisation of the collateral anatomy from an interventional perspective within a CTO population. We aimed to define both the anatomy and interventional utility of collaterals within a CTO population, expanding on the preceding work and providing a frame of reference for interventional cardiologists working within the subspecialty of CTO percutaneous coronary intervention (PCI).
Methods
We studied the coronary angiograms of patients referred for consideration of CTO PCI at six tertiary referral centres in the United Kingdom (Edinburgh Heart Centre, Golden Jubilee National Hospital Glasgow, Freeman Hospital Newcastle-upon-Tyne, Ninewells Hospital Dundee, Bristol Royal Infirmary, and Belfast Health & Social Care Trust) over a four-year period between November 2010 and November 2014.
Baseline demographics including gender, age and previous history of coronary artery bypass graft (CABG) surgery were documented.
Coronary angiograms were analysed by interventional cardiologists specialising in CTO PCI. All angiograms were performed electively on patients with stable coronary artery disease. A CTO was defined as a lesion with Thrombolysis In Myocardial Infarction (TIMI) grade 0 flow within the occluded segment and angiographic or clinical evidence (or a high likelihood) of an occlusion duration ≥3 months. Each lesion was further stratified according to the major epicardial artery involved and according to coronary dominance. All visible collaterals with collateral connection grade ≥1 (CC1) were defined and described5. The presence of bridging collaterals was also noted.
A subgroup of CTOs (n=277) was assessed to determine whether the collateral supply would permit retrograde access to the occluded vessel. This was defined as the presence of at least one collateral channel which, from angiographic appearances alone, was considered suitable to deliver interventional equipment from the donor vessel to the target vessel beyond the occluded segment by an operator experienced in retrograde CTO techniques. The primary criterion applied, in the assessment of interventional suitability, was the operator’s previous experience of collateral channel crossing. Other defining criteria included:
Type of collateral: septal, epicardial, or bypass graft.
Channel tortuosity: defined as the presence of ≥2 high-frequency, successive curves (within 2 mm) in the context of epicardial collaterals and ≥1 high-frequency curve that failed to uncoil in diastole for septal channels (thus a measure of channel distensibilty). A high-frequency curve is defined as a curve that is >180 degrees occurring within a segment length <3 times the diameter of the collateral.
Channel size: defined as being unlikely to permit the passage of a microcatheter without significant risk of collateral injury, generally <1 mm diameter.
Channel length: defined as a long collateral channel that would be difficult to negotiate with equipment, even with the use of a shortened guide and mother-daughter catheter.
Channel entry: adverse channel entry angle, typically defined as <45 degrees (Figure 1), or disease/previous stent at channel entry.
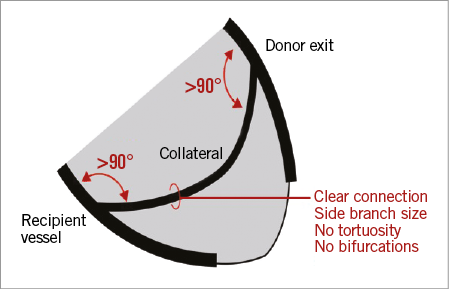
Figure 1. An optimal collateral channel.
Channel exit: adverse channel exit angle, typically defined as <45 degrees (Figure 1), disease at channel exit, or channel exit close to/at distal cap.
Multiple bifurcations: particularly at points of marked curvature, or just after collateral channel entry.
Risk: a collateral channel thought to be at high risk of damage, or traversing it anticipated to result in adverse clinical sequelae (such as ischaemia caused by traversing a very dominant collateral channel). We defined a collateral at high risk of damage as an epicardial collateral ≤ half the diameter of the microcatheter.
The kappa statistic for intra- and inter-observer variability was calculated. Categorical variables were compared using the Fisher’s exact test with a two-sided test.
Results
Coronary angiograms from 481 patients with 519 CTOs were analysed. Consistent with previous population studies, CTOs most commonly involved the right coronary artery (RCA; n=279, 53.8%), then the left anterior descending (LAD) artery (n=153, 29.5%), and least frequently the circumflex (Cx) artery (n=87, 16.8%). A right dominant circulation was present in 90.4% of patients. Mean age was 64.5 (range 27-93) years, 19.1% (n=92) were female, and 13.3% (n=64) had previously undergone CABG surgery.
Right coronary artery chronic total occlusion (RCA CTO)
There were 279 CTOs of the RCA, of which 275 (98.6%) were right dominant. A total of 591 collaterals (mean 2.1 collateral pathways per CTO) were identified with 20 discrete filling patterns. These are described below with the most common pathways illustrated in Figure 2 and an angiographic example in Figure 3.
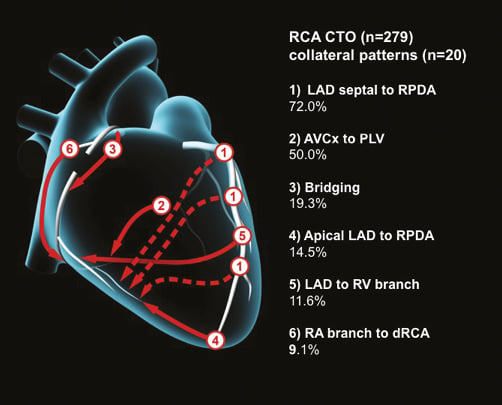
Figure 2. Common collateral patterns in RCA CTOs.
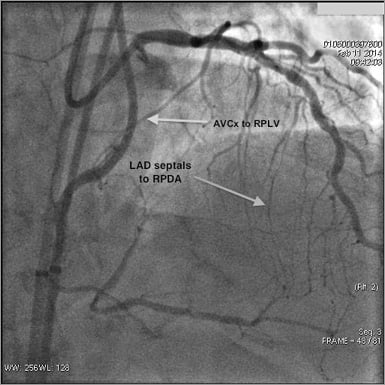
Figure 3. Angiographic example of collateral patterns in an RCA CTO.
198 (72.0%) of right dominant CTOs had collaterals from LAD septals to the right posterior descending artery (RPDA).
129 (46.9%) had collaterals from the atrioventricular Cx (AVCx) artery to the posterolateral ventricular (PLV) branch of the RCA. This pattern was also seen in 2/4 (50%) with a left dominant circulation.
53 (19.3%) had bridging collaterals. Bridging collaterals were also seen in 1/4 (25%) with a left dominant circulation.
40 (14.5%) had an epicardial collateral from the apical LAD to the RPDA.
32 (11.6%) had collaterals from the LAD to the right ventricular (RV) branch.
25 (9.1%) had collaterals from the right atrial (RA) branch to the distal RCA.
23 (8.4%) had collaterals from the obtuse marginal (OM) branch to the PLV branch.
22 (8.0%) had collaterals from the OM to the RPDA.
16 (5.8%) had collaterals from the RV branch to the RPDA.
14 (5.1%) had collaterals from LAD septals to the PLV. This was also seen in 1/4 (25%) with left dominant circulation.
11 (4.0%) had collaterals from a diagonal branch to the PLV.
8 (2.9%) had collaterals from an LAD diagonal to the RPDA.
Less frequently encountered collaterals were seen from the conus branch (CB) to the RV branch (n=5), sinus node (SN) branch to the RPDA (n=4), RV branch to the PLV (n=3), RA branch to RV branch (n=3), as well as single collaterals from the RPDA to the PLV, SN branch to the PLV, and ramus intermedius to the PLV.
On further analysis of a subgroup of the RCA CTOs (n=87), in the absence of visible LAD septal collaterals to the RPDA, there was a distal LAD epicardial collateral to the RPDA in 50% of CTOs, compared to 3.5% when septal collaterals were present (p<0.0001). The prevalence of AVCx to PLV, OM to PLV, RA to distal RCA, and bridging collaterals was similar in the presence or absence of visible septal collaterals.
Left anterior descending chronic total occlusion (LAD CTO)
There were 153 CTOs of the LAD, of which 129 (84.3%) were in right dominant circulations. A total of 299 collaterals (mean 2.0 collateral pathways per CTO) were identified with 13 discrete filling patterns. These are described below with the most common pathways illustrated in Figure 4 and an angiographic example in Figure 5.
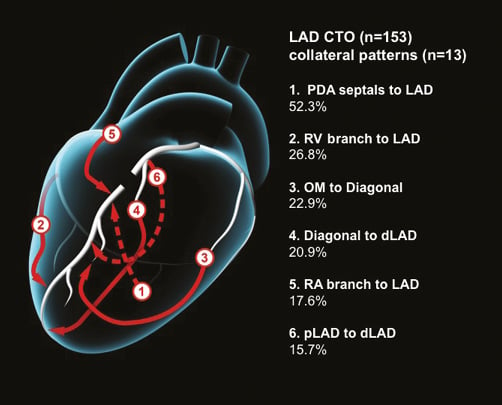
Figure 4. Common collateral patterns in LAD CTOs.
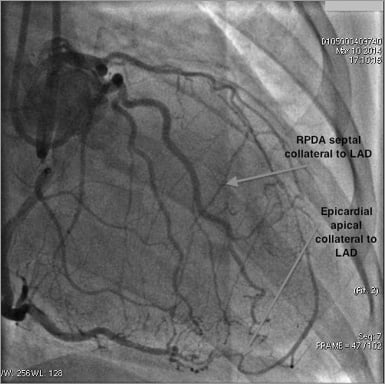
Figure 5. Angiographic example of collateral patterns in a LAD CTO.
80 (52.3%) of all LAD CTOs had collaterals from PDA septals to the LAD. These were present in 70/129 (54.3%) CTOs with right dominant, and 10/24 (41.7%) with left or co-dominant circulations.
41 (26.8%) had RV branch to LAD collaterals. Of these, 32/129 (24.8%) were right dominant and 9/24 (37.5%) left dominant.
35 (22.9%) had OM to diagonal collaterals. Of these, 31/129 (24.0%) were right dominant and 4/24 (16.7%) left dominant.
32 (20.9%) had diagonal branch collaterals to a more distal segment of the LAD (auto-collateral). Of these, 27/129 (20.9%) were right dominant and 5/24 (20.8%) left dominant.
27 (17.6%) had RA branch to proximal LAD collaterals. Of these, 17/129 (13.2%) were right dominant and 10/24 (41.7%) left dominant.
24 (15.7%) had proximal LAD septal to more distal septal collaterals (auto-collateral). Of these, 21/129 (16.3%) were right dominant and 3/24 (12.5%) left dominant.
20 (13.1%) had RPDA to apical LAD. Of these, 17/129 (13.2%) were right dominant and 3/24 (12.5%) left dominant.
17 (11.1%) had bridging collaterals. Of these, 15/129 (11.6%) were right dominant and 2/24 (8.3%) left dominant.
9 (5.9%) had CB to LAD collaterals. These were all right dominant.
6 (3.9%) had PLV to LAD collaterals. Of these, 4/129 (3.1%) were right dominant and 2/24 (8.3%) left dominant.
Less frequently encountered collaterals were seen from ramus intermedius to diagonal (n=3), Cx to LAD (n=2), and SN branch to LAD via septals (n=1).
On further analysis of a subgroup of the LAD CTOs (n=45), in the absence of PDA septal collaterals to the LAD, diagonal to more distal diagonal collaterals were more common (40.9% vs. 13.0%, p=0.047). OM to diagonal collaterals were non-significantly more common (45.4% vs. 30.4%, p=0.365), while the prevalence of RA, RV, or PDA epicardial collaterals to the LAD was similar in the presence or absence of septal collaterals.
Circumflex chronic total occlusion (Cx CTO)
There were 87 CTOs of the circumflex artery (Cx), of which 76 (87.4%) were in right dominant circulations. A total of 120 collaterals (mean 1.4 collateral pathways per CTO) were identified, with 12 discrete filling patterns. These are described below with the most common pathways illustrated in Figure 6 and an angiographic example in Figure 7.
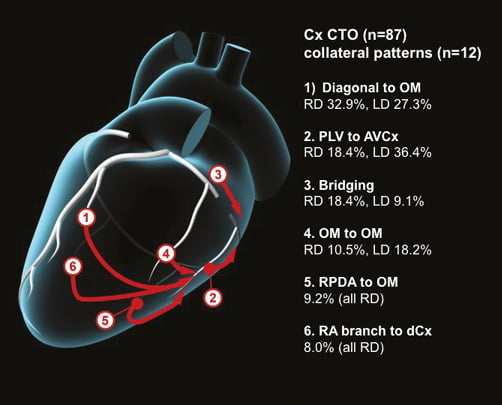
Figure 6. Common collateral patterns in Cx CTOs.
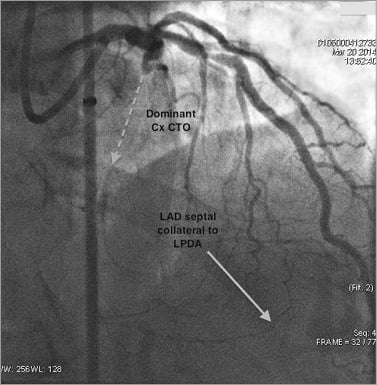
Figure 7. Angiographic example of collateral patterns in a Cx CTO.
28 (32.2%) of all Cx CTOs had collaterals from the diagonal branch of the LAD to the OM branch of the Cx. Of these, 25/76 (32.9%) were right dominant and 3/11 (27.3%) left dominant.
18 (20.7%) had PLV to AVCx collaterals. Of these, 14/76 (18.4%) were right dominant and 4/11 (36.4%) left dominant.
15 (17.2%) had bridging collaterals. Of these, 14/76 (18.4%) were right dominant and 1/11 (9.1%) left dominant.
10 (11.5%) had proximal OM branch to more distal OM branch (auto-collaterals). Of these, 8/76 (10.5%) were right dominant and 2/11 (18.2%) left dominant.
8 (9.2%) had RPDA to OM branch collaterals (all right dominant).
7 (8.0%) had RA branch to distal circumflex collaterals (all right dominant).
7 (8.0%) had apical LAD to OM collaterals (all right dominant).
6 (6.9%) had ramus intermedius to OM branch collaterals (all right dominant).
6 (6.9%) had collaterals from proximal Cx to more distal portion of Cx (all right dominant).
5 (5.7%) had LAD septal to OM collaterals. Of these, 4/76 (5.3%) were right dominant and 1/11 (9.1%) left dominant.
5 (5.7%) had LAD septal to LPDA collaterals (all left dominant).
5 (5.7%) had PLV branch to OM collaterals (all right dominant).
Interventional collaterals
In more than half of the overall population (277/519 CTOs, 263/481 patients) collaterals were assessed for interventional potential. A CTO was defined as having “interventional collaterals” if at least one collateral within the total number of collaterals supplying the occluded vessel was considered to have interventional potential. At least one interventional collateral was noted in 167 patients (63.5%). Intra-observer agreement was excellent (kappa statistic of 0.92 [SE 0.14]) and inter-observer agreement was good (0.77 [SE 0.15]). The interventional suitability of each of the collateral pathways is reported alongside the prevalence of each pathway in Table 1A, Table 1B, Table 1C.
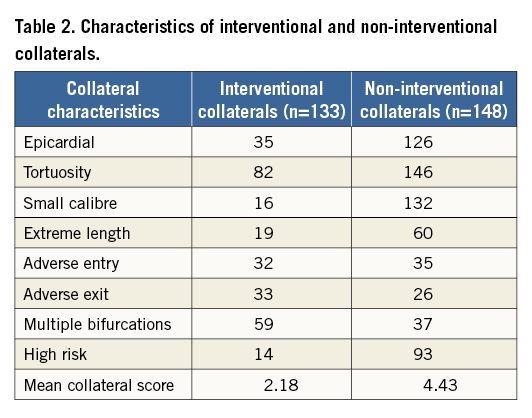
The characteristics of interventional and non-interventional collaterals are further described in a subgroup of these CTOs (n=166) (Table 2). Using a novel collateral scoring system, with one point assigned for each of the adverse characteristics defined earlier (epicardial channel, channel tortuosity, small channel size, channel length, adverse channel entry, adverse channel exit, multiple bifurcations, and high risk), interventional (n=133) and non-interventional collaterals (n=148) had mean scores of 2.18 and 4.43, respectively.
Within this subgroup of CTOs (n=166), 114 (68.7%) had at least one interventional collateral and 52 (31.3%) had non-interventional collaterals only. Of the 114 CTOs with an interventional collateral, 53 (46.5%) had a retrograde attempt, which was successful in 86.8% of cases. Of the 52 CTOs with non-interventional collaterals, only three (5.7%) had a retrograde attempt, none of which was successful. Interventional collateral analysis was from CTO-specific angiography in 91.6% and diagnostic angiography in 8.4%. Angiography was performed by dual injection in 69.2% of CTOs and single-injection angiography in 30.8%. The reason given for single-injection angiography was the presence of only ipsilateral collaterals (59.6%), analysis of a diagnostic angiogram only (28.8%), or a J-CTO score of 0 (11.5%). A field of view of 20 cm, 17 cm or 15 cm was used in 50.0%, 19.9%, and 30.1%, respectively. Variable magnification was used in many cases with the smallest field of view reported. There was no intensifier panning during collateral analysis image acquisitions in 88.6% of CTOs. Donor vessel catheter sizes were 5 Fr, 6 Fr, 7 Fr and 8 Fr in 7.8%, 44.0%, 26.5%, and 21.7%, respectively.
Discussion
Evidence from observational studies demonstrates that successful CTO PCI can result in durable improvement in anginal symptoms, quality of life and left ventricular function6-9. Whilst uptake of CTO PCI was previously hindered by poor procedural success rates, the evolution of techniques, including the retrograde approach, has been associated with improved outcomes, despite increasing case complexity, and thus has resulted in increasing numbers of CTO procedures being performed10-15.
In recent years the role of the collateral circulation in maintaining myocardial viability has become more apparent1-4. Refinement of retrograde techniques and equipment now means that the collateral circulation is a practical route to access the distal coronary bed beyond the occlusive segment efficiently, rather than simply a means of its visualisation10-15. It is therefore of critical importance that the precise anatomy of these collaterals is defined to facilitate case planning16. Despite this, knowledge and reporting of collateral anatomy has lagged behind physiological studies.
Levin’s landmark paper, published over forty years ago, described patterns of collateral blood supply in a relatively modest number of subjects with both stenotic and occluded vessels17. Our contemporary analysis assesses collateral filling patterns in a significantly larger cohort of patients and includes only those with CTOs, with their increased propensity for collateralisation18. It is likely that the observed differences in collateral patterns from those previously described reflect not only the different patient populations, but also changes in imaging technology, and the contemporary CTO interventional perspective. It is noteworthy that the original assessment by Levin was carried out without reference to cranial or caudal angulations, which may partly explain the lower number of collateral patterns described and the failure to identify some important collateral pathways. It is also important to consider that, while imaging technology and techniques have improved, contemporary image resolution at very small dimensions (~0.2 mm per pixel) is limited in comparison to cine film, which has no such limit.
It is important to note that in this study only angiographically visible collaterals were described (CC≥1). This is of particular relevance in the assessment of “interventional” collaterals in the septal circulation where “invisible” (CC0) collaterals can occasionally be used successfully11.
While patient factors and operator preference result in some variability, we recommend a gold standard approach to imaging potential interventional collaterals: dual-injection angiography, ≥7 Fr donor catheter size, ≥20 cm field of view, ≥15 fps frame rate, with no panning of the image intensifier.
RCA CTO collaterals
Our study confirms that collaterals from LAD septals to the RPDA and from the AVCx to the PLV branch of the RCA are the most common in RCA CTOs. While the original work from Levin found the same patterns, the absolute incidence was considerably higher in our study (72% versus 38% of LAD septals to the RPDA, and 47% versus 23% of AVCx to PLV collaterals). Indeed, the high incidence of these two collateral patterns has important implications for the feasibility of retrograde access. Other notable observations were that collaterals from the OM to the PLV were less frequent in our analysis (8% versus 16%), and that there were three collateral patterns reported in our population not described by Levin: OM branch to the PDA (8%), LAD septals to the PLV (5%), and diagonal branch to the PLV (4%)17.
LAD CTO collaterals
In the LAD CTOs we also found a high frequency of septal collaterals from the RPDA (or LPDA in the small group of left dominant circulations) to the LAD. While this was the most common LAD collateral pattern seen in our study (52%), Levin only found these in 2% (three patients), a difference unlikely to be explained solely by differences in populations. The previous underrecognition of this, the most important collateral pathway for retrograde treatment of LAD CTOs, is a key finding of our study. Of note, there were significantly more septal collaterals from the LAD to PDA than PDA to LAD, posing the question of whether recruitment of septal collaterals from the PDA by the LAD requires the presence of more severe disease than in the reverse direction. The relative frequencies of the other most common collateral filling patterns were broadly similar to those described by Levin. Either an RV marginal or RA branch collateral to LAD was present in 44%, and LAD septal auto-collaterals in 16% of CTOs. Only the PLV to LAD collaterals, seen in 4% of our LAD CTOs, were not previously described17.
Cx CTO collaterals
There was a lower frequency of CTOs of the Cx artery within our study population, the reason for which remains uncertain. This may reflect a truly lower incidence, or that Cx CTOs are more likely to be clinically silent, present atypically, are more commonly managed with medical therapy, or that CABG is more often recommended if the Cx CTO is in a left dominant circulation.
The 120 collaterals we describe in 87 Cx CTOs represent nearly sixfold more than in the Levin analysis (n=21). With such modest numbers we are cautious in making comparisons, but note that, while Levin described five collateral patterns, we observed fourteen distinct patterns. Importantly, we found that Cx CTOs had a lower number of collateral filling pathways (mean 1.4 per CTO) than RCA and LAD CTOs (2.1 and 2.0, respectively). In addition, the most frequent Cx CTO collateral (diagonal to OM branch) was only seen in 32% of occlusions. It is interesting to speculate whether this lower frequency of a wide variety of predominantly epicardial collaterals may explain the lower procedural success rates in CTOs of the Cx treated by a retrograde approach19.
Our detailed description of CTO collateral anatomy can facilitate directed angiographic analysis, procedural planning and CTO PCI training: for an RCA CTO, the focus should be on the utility of LAD septal to RPDA and AVCx to RPLV collaterals; for an LAD CTO, the PDA septal to LAD, LAD septal auto-collateral, and RV marginal and RA branch to LAD collaterals; and for a Cx CTO, LAD septals to LPDA or OM, PLV to AVCx, and diagonal to OM collaterals.
Interventional collaterals
While retrograde access has made recanalisation of longer, more complex CTOs feasible, there remain significant limitations, with the most common failure mode being an inability to cross the collaterals19. In contemporary literature a failure rate of up to 20% is reported, though the degree of any selection bias involved is uncertain (i.e., what proportion of collaterals are deemed unsuitable and never attempted)20. We have attempted to address this limitation by performing a visual assessment of collateral channel crossing feasibility to provide an insight into what subset of CTOs would be suitable for retrograde access. We found that 64% of CTOs were supplied by at least one collateral that appeared suitable for use as part of a retrograde procedure. Allowing for a failure rate of 20% in collateral crossing, this would imply that ~50% of CTOs could be treated by a retrograde approach. Even with the possibility of being able to cross very small, or angiographically invisible, septal collaterals11, this still implies that interventional strategies other than retrograde will be necessary to maintain high procedural success rates.
In an attempt to quantify the visual assessment of collateral channels we devised a novel scoring system, calculated from readily identifiable adverse characteristics. Some characteristics were more discriminating than others between what we anticipated to be “interventional” or “non-interventional collaterals” (Table 2). The composition and potential usefulness of a collateral score as a predictor of collateral channel crossing and retrograde procedural success will require further validation.
Both a strength and limitation of this study is that the analysis was of angiograms performed by both specialist CTO operators and non-CTO operators. Thus, there was a combination of single- and dual-injection angiography with variability in catheter size and use of cine magnification and panning, reflective of real-life practice. Angiographic assessment is subjective; however, despite this, we demonstrated good inter-observer agreement. We acknowledge that this is not an exhaustive definition of collateral anatomy, but reflects angiographically visible collaterals. Furthermore, it has been suggested that some collateral pathways are permanent and increase in size according to perfusion gradients, hence the presence of recruitable collaterals in a subpopulation of patients presenting with acute myocardial infarction21. Some evidence of a similar phenomenon was seen in our population with less visible septal collateralisation in the presence of a dominant epicardial collateral.
Conclusions
This is the largest analysis of collateral anatomy in patients with CTOs, comprehensively describing the prevalence of all visible collateral pathways. The assessment of collateral characteristics and anticipated interventional utility provides useful knowledge for selecting interventional strategies during CTO PCI procedure planning.
| Impact on daily practice This is the first study to describe definitively the anatomical characteristics of the coronary collateral circulation within a large CTO population. This will be of significant benefit when planning retrograde CTO PCI. Furthermore, knowledge of the most common and usable collaterals will allow training to focus on appropriate crossing techniques, improving procedural efficiency and the targeted development of future technology. |
Acknowledgements
We wish to acknowledge Adrian Brown and Optima Education for assistance with the illustrations.
Conflict of interest statement
The authors have no conflicts of interest to declare.

