
The use of medical devices (MDs) has impacted positively on quality of life and survival. Prosthetic heart valves (PV) are among the most implanted blood-contacting MDs. About four million heart valve replacements have been performed over the past 50 years, and this remains the only definitive treatment for most patients with severe valvular heart disease. About 300,000 PV implantations are performed every year worldwide; the total number of valve replacements is projected to be 850,000 per year by 20501. With the advent of transcatheter aortic valve implantation (TAVI), these figures are expected to increase even more. Two types of PV are in use today to fulfil this growing demand, the mechanical valve and the tissue valve, each with its inherent assets and drawbacks. Mechanical PV, made from synthetic materials (pyrolytic carbon combined with metallic and polymeric components), display unnatural haemodynamics and are prone to thrombus formation, which necessitates lifelong anticoagulation therapy, with the concomitant increase in bleeding risk. Biological PV, derived from human cadavers or, most often, from glutaraldehyde-fixed and decellularised porcine or bovine cardiac tissue, are less thrombogenic, but they have a shorter lifespan due to calcification and tearing. Calcification of PV is now thought to be an active process that involves dysregulated mineral metabolism, lipid-mediated inflammation as well as the coordinated actions of several cell types, e.g., circulating endothelial cells, residual fibroblastic cells, infiltrating inflammatory and immune cells, and bone marrow-derived progenitors2. In any case, the colonisation of PV by bacteria or fungi at the moment of implantation or long after (haematogenous infection) can cause endocarditis. PV endocarditis (PVE) is the most severe form of infective endocarditis, which occurs in 1 to 6% of patients with PV and affects mechanical and bioprosthetic valves equally3. PVE is a deadly disease with in-hospital mortality of up to 20-40%. The infection usually initiates at the junction between the sewing ring and the annulus, leading to perivalvular abscess, dehiscence, pseudoaneurysms and fistulae, or is located on the leaflets of the prosthesis, which forms vegetations and results in cusp rupture and perforation. Staphylococci and enterococci are the most frequently incriminated microorganisms. Elimination of the infection is highly challenging due to biofilm formation on the surface of the PV and the high frequency of antibiotic resistance; in such cases, the removal of the MD is often the sole solution. Since thrombosis and infection are inter-related processes, involving interactions between platelets and bacteria, bacteria and plasma factors or fibrin, it is therefore crucial to develop strategies that prevent both thrombosis and infection when considering blood-contacting devices, in particular intravascular MDs and PV. The adage that prevention is better than cure is thus very apt for PV.
Advances in surface enhancement, relating to improved haemocompatibility, along with antibacterial and antibiofilm characteristics to fight against PV healthcare-associated infections, thrombosis, and degeneration, represent current research and development challenges4. For over 50 years, biological tissue fixation with glutaraldehyde has been the method of choice for the preparation of PV. However, cytotoxic residual aldehydes contribute to the generation of unwanted immunogenicity. The surface of glutaraldehyde-fixed PV does not reproduce the antithrombotic and anti-infective properties of native valve endothelia, and both calcification and structural valve deterioration have proven to be major PV drawbacks. Alternative tissue engineering approaches to prevent PV deterioration have thus progressively emerged but without providing a definitive solution. They include improved post-fixation (i.e., with monosodium glutamate) treatment of glutaraldehyde pre-treated PV, additional chemical treatments with anti-calcification agents (i.e., amino oleic acid), the use of alternative fixing agents (e.g., phytic acid, polyepoxy compounds, carbodiimide), and the development of novel methods based on the post-implantation re-endothelialisation of a PV resorbable matrix with the patient’s circulating endothelial progenitor cells. These last technologies, although more in the distant future, are nonetheless very attractive. The use of modern coating methods, which can serve as a vector for the functionalisation of glutaraldehyde-fixed PV, is also very promising. Thanks to a European Research Council (ERC) Consolidator grant (2015-2020, PV-COAT), we have developed, at the GIGA Cardiovascular Science Unit of the University of Liège, a new polymeric bioactive coating for PV endowed with antifouling, antiplatelet, anticoagulant and antimicrobial properties (Figure 1). This bioactive coating is made up of a multilayer of cross-linked polymeric nanogels loaded with releasable small molecules, referred to as nanoreservoirs (WO2018/122318 A1). Nanoreservoirs are deposited on a surface pre-coated with 3,4-dihydroxy-L-phenylalanine (DOPA) units and polyallylamine hydrochloride (PAH). Nanogels (100-200 nm) are formed by covalent reaction between an oxidised homopolymer of methacrylamide DOPA and PAH. Loaded nanoreservoirs are obtained by forming the nanogels in the presence of bioactive molecules. In the present case, we made use of ticagrelor. Ticagrelor has the advantage, beyond that of being a powerful antiplatelet agent, of having antibacterial and antibiofilm properties against methicillin-sensitive and resistant S. aureus and multidrug-resistant enterococci, without itself inducing resistance5 (EP3292867 B1). Hydrophilic polyethylene glycol (PEG) is grafted on the top of multilayered nanogels. The final ticagrelor-loaded bioactive coating is named Triafluogel. This technology was applied, for the first time, to a Mosaic® biological valve (model 305, 19 mm; Medtronic, Minneapolis, MN, USA) (Figure 1). The haemodynamic performance of the coated PV was tested in a Pulse Duplicator (ViVitro Labs, Victoria, BC, Canada) in accordance with the ISO-5840 heart valve testing standard (Lancellotti P. Innovations in TAVI: new valves, new procedures. Bioactive surface coating for bioprosthetic heart valve: Improved medical device biocompatibility. Presented at EuroPCR, Paris, France, 23 May 2019). The recommended FDA target for valve durability of 200 million cycles was achieved over a six-month period by using a HiCycle Durability tester producing 800 open/closure cycles per minute (ViVitro Labs). Under 5 L/min flow, 70 bpm of cardiac frequency, and 100 mmHg forward pressure, the haemodynamic performance of the coated valve, examined every 50 million cycles, presented similar characteristics in terms of regurgitant fraction, effective orifice area, and transvalvular gradient to those of a non-coated Mosaic biological valve (Figure 1). After 200 million cycles (six-year life), the modification of tissue valve surface remained visible by second harmonic generation (SHG) microscopy (Figure 1). To examine the antibacterial properties of Triafluogel-modified PV, leaflet pieces of coated and non-coated Mosaic PV were incubated for 24 hours with S. aureus (ATCC 25904). In contrast to non-coated PV, no bacteria were able to grow on the surface of coated PV, as shown by scanning electron microscopy. The antithrombotic properties of Triafluogel were demonstrated at 1,800 s-1 shear rate (i.e., mean systolic shear stress on aortic valve leaflets) in a cone-and-plate viscometer (Impact-R™; Matis Medical, Beersel, Belgium). Shear stress-induced platelet adhesion and aggregation on coated wells were strongly inhibited as compared to non-coated surfaces (Figure 1). By dramatically improving the haemocompatibility of PV without altering its haemodynamic performance, our durable bioactive coating therefore represents a major breakthrough. Of note, this coating can also be attached onto any prosthetic material, whether biological such as bovine pericardium or inert, including plastic such as polyurethane (composing catheters) and metal such as titanium (the main component of pacemakers). Therefore, our bioactive coating might be used to improve the clinical performance of most MDs. Its application for the prevention of catheter infection and thrombosis is currently being assessed with the help of an ERC Proof of Concept grant (2019-2020).
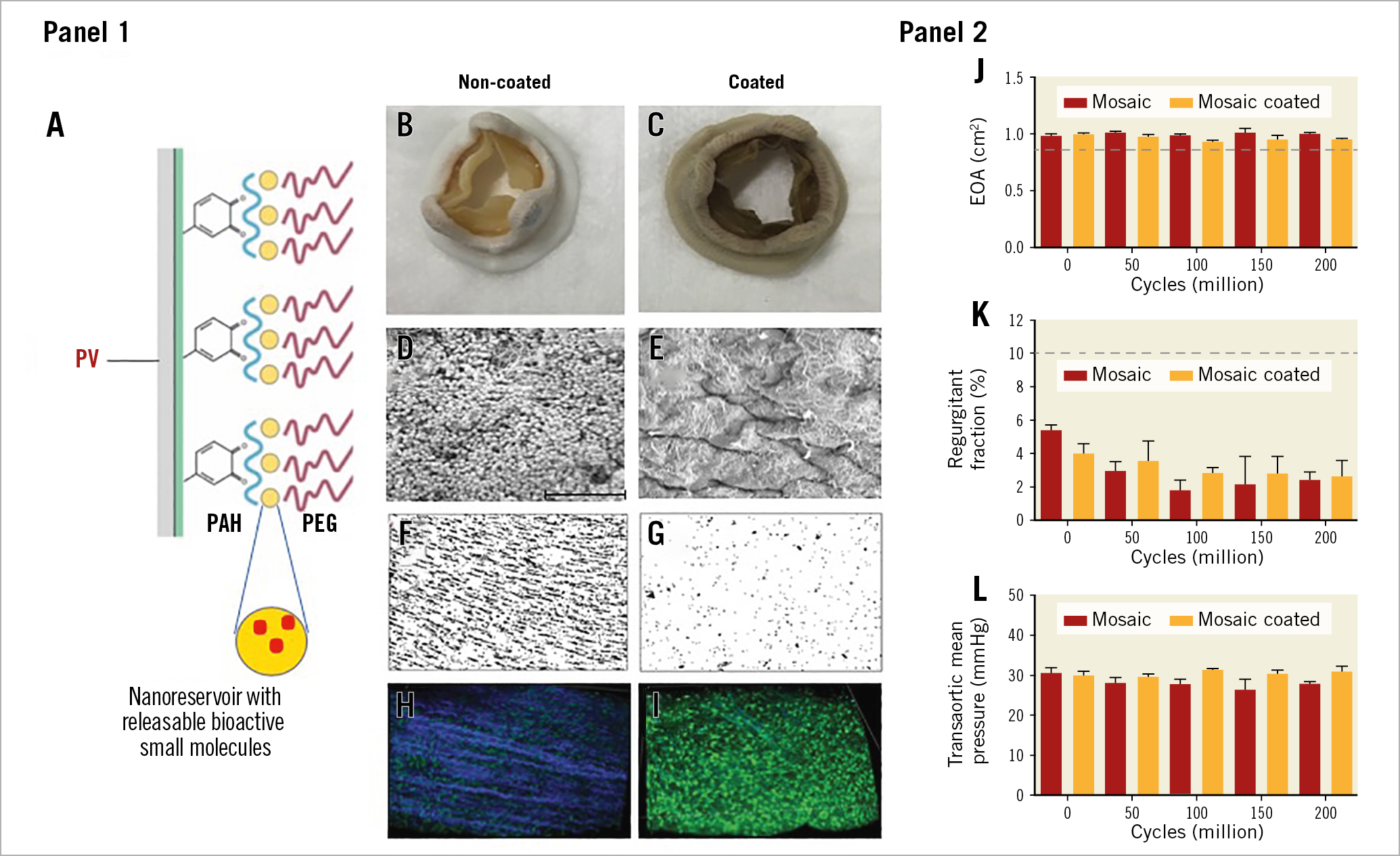
Figure 1. Medtronic Mosaic coated prosthetic valve: haemocompatibility, antibacterial properties and durability testing. Panel 1. Schematic representation of our bioactive polymeric coating (A), Medtronic Mosaic PV before (B) and after (C) coating procedure. SEM analysis of non-coated (D) and Triafluogel-coated (E) pieces of Mosaic valve leaflets after 24-hour incubation with S. aureus. Bar=10 µm. Analysis of platelet adhesion and aggregation on non-coated (F) and Triafluogel-coated (G) Impact-R wells. Visualisation of modified Mosaic PV surface by SHG microscopy after 200 million cycles in a HiCycle Durability tester (H, non-coated PV, collagen fibres appear in blue) (I, coated PV). Panel 2. Haemodynamic performance of coated and non-coated Mosaic PV (J, effective orifice area [EOA]; K, total regurgitant fraction; L, transvalvular mean pressure). Dashed lines represent the minimal performance for a 19 mm PV.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

