Abstract
Aims: To compare the outcomes of drug-eluting (DES) vs. bare-metal (BMS) stents for stenting of native aorto-ostial lesions (AOL) and to identify predictors of major adverse cardio and cerebrovascular events (MACCE).
Methods and results: A total of 181 patients (182 AOL) who underwent stenting of AOL were retrospectively identified: right-coronary artery in 130 (71.4%), left main in 52 (28.6%). In-hospital event rate was 1.1% (two non-Q-wave myocardial infarctions). Follow-up was possible in 98.3%, median time=23.9 months (IQR 12.1-37.7). Event rates and survival MACCE-free were not significantly different between DES and BMS. After multivariate analysis, only the logistic EuroSCORE >10% predicted MACCE (HR=4.66, 95% CI: 2.38-9.12, p<0.001), whereas the predictors for TLR were age (HR=0.96, 95% CI: 0.92-1.00, p=0.039) and the stented artery (RCA vs. LM, HR=10.2, 95% CI: 1.37-75.45, p=0.024).
Conclusions: AOL stenting can be performed with high success and low complication rates. At follow-up, no significant differences in event rates were found between DES and BMS; EuroSCORE>10% was the only predictor of MACCE.
Introduction
Percutaneous coronary intervention (PCI) of aorto-ostial lesions (AOL) is always challenging for the interventional cardiologist. Abundant elastic fibres and lesion rigidity1-3 are classically described as the main features determining the outcome after standard balloon angioplasty and leading to more procedure-related complications4-6. Laser therapy7 followed by balloon angioplasty in selected cases and rotational atherectomy8 with stenting as a bail-out strategy, were evaluated initially in the pre- “routine stenting” era. Greater acute gain and lower restenosis rates in AOL were achieved after stent implantation in comparison with conventional balloon angioplasty or debulking therapy alone9. However, restenosis rates remained elevated when compared with non-ostial lesions10,11. Later, drug-eluting stents (DES), evaluated either alone12 or in comparison with bare-metal stents (BMS)13-15, showed more favourable results in reducing revascularisation rates. The advantages of directional atherectomy have recently been reviewed with examples of AOL16.
In a recent registry of “‘real world” unselected population treated with DES, AOL prevalence was only 2.6%17. The low prevalence and anatomical considerations render it difficult to study in randomised trials, with the information regarding AOL available only from registries with a limited number of patients. We therefore sought to review AOL treated by stenting in our centre. The main goals of our study were: to compare event rates between DES and BMS and to identify predictors of long-term major adverse cardiac and cerebrovascular events (MACCE).
Methods
Study population
We retrospectively studied 181 consecutive patients (182 lesions) who underwent PCI with stent placement for AOL between November 2004 and February 2009 in our centre database. An AOL was defined as >50% stenosis by visual estimation involving the coronary ostium, arising within 3mm of the aortic orifice of the right coronary (RCA) artery or left main (LM), visualised in the best angiographic position without foreshortening. Only native, denovo AOL were considered, with patients presenting with symptomatic stable angina, ST-elevation MI (STEMI), acute coronary syndromes (unstable angina or non-ST elevation MI), or silent ischaemia. In the case of LM stenting, we excluded patients with concomitant distal LM (bifurcation) disease. Patients with significant valvular disease or cardiomyopathy were also excluded from the study.
Intervention and periprocedural medication
Procedural techniques involving PCI of AOL lesions have recently been published18. Stenting was performed according to standard techniques. Multiple angiographic views were obtained before stent implantation to place the stent correctly, trying to achieve 1mm protrusion into the aorta, inflating at high pressure (>14atm). Balloon pre-dilation, debulking techniques or stent post-dilation using non-compliant balloons were at the operator’s discretion. All patients were pre-treated with a loading dose of 300-600mg clopidogrel and 100mg aspirin unless they were taking chronic therapy. After the procedure, at least 75mg of aspirin was prescribed lifelong and 75mg of clopidogrel for at least one month after BMS and one year after DES. Intravenous heparin (70U/Kg) was given during the procedure. Adjunctive treatment with IIb/IIIa inhibitors was given at the operator’s discretion. Creatine kinase (CK) and CK-MB were systematically measured in all patients at three, six and 12h after procedure. Electrocardiography (ECG) was performed after PCI, before discharge, or in response to patients’ symptoms.
Angiographic analysis
Standard quantitative analysis was performed using the guiding catheter as calibration. Quantitative coronary angiography (QCA) was generated by the computer-based coronary angiography analyses system (CAAS II; Pie Medical Imaging, Maastricht, The Netherlands). Minimal luminal diameter (MLD), reference vessel diameter (RVD) and percent diameter stenosis were measured before and after the procedure. Acute gain was defined as the difference in MLD before and after stenting. In-stent restenosis (ISR) was defined as >50% stenosis within a stented segment at follow-up, including 5mm edges.
Definitions and follow-up
Immediate angiographic success was defined as distal TIMI3 flow in the stented vessel with a final residual stenosis less than 20%. During hospitalisation, events were assessed as death, myocardial infarction (MI), cerebrovascular accident (CVA), new PCI, or emergency coronary artery by-pass grafting (CABG). At follow-up, MACCE was defined as cardiac death, MI, target lesion revascularisation (TLR), or CVA. Death was defined as cardiac unless clearly attributed to another cause. MI was defined as either the presence of new Q-wave in two contiguous leads in the ECG or elevation of CK-MB ≥2x upper limit, post-procedural or at follow-up. TLR was defined as repeat revascularisation either percutaneous or surgically of the stented segment (including 5mm edges). CVA was defined as transient or permanent neurological deficit, supported by brain imaging or by a neurologist consultation. Stent thrombosis was assessed by the Academic Research Consortium criteria defined as definite, probable, or possible19. Renal failure was defined by a creatinine clearance (Cockroft-Gault formula) <60 ml/min, multivessel disease was defined by more than one epicardial coronary artery with >70% stenosis by visual estimation.
Clinical follow-up was performed by office visits to or telephone interviews with the attending cardiologist, general physician, or directly with the patient. Routine follow-up angiography was not performed. Repeat angiography was performed in the event of recurrent anginal symptoms, silent ischaemia detected by stress test, scintigraphy, stress echocardiography, or after multidetector computed tomography, although a small number of asymptomatic patients also had repeat angiography.
Statistical analysis
Categorical variables are expressed as frequency (%) and compared using Chi-Square or Fisher´s exact test. Continuous variables are expressed as mean (±standard deviation [SD]) or median (±interquartile range [IQR]), as appropriate, and comparisons were made using the independent samples t-test. The Cox proportional hazards model was generated to compare event-free survival between BMS and DES adjusted for possible confounders (variables with p<0.1 in the univariate analyses). A stepwise procedure was used to identify independent predictors for MACCE, TLR, and cardiac death at follow-up. A p-value<0.05 was considered statistically significant for all the comparisons. All analysis was performed with PASW statistics version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline clinical and procedure data
Baseline demographic, clinical, and angiographic characteristics stratified by the type of stent are presented in Table1. A total of 181 patients (182 lesions due to RCA and LM ostia in the same patient) were identified: RCA in 130 (71.4%), LM in 52 (28.6%). Thirty patients (16.6%) had an ejection fraction <50%, 45 patients (24.9%) had a EuroSCORE (logistic)>10%, 92 (50.8%) had creatinine clearance <60ml/min (84 [46.4%] had between ≥30ml/min and <60ml/min, eight [4.4%] had <30ml/min). Eighty-seven (47.8%) BMS and 95 (52.2%) DES were implanted.
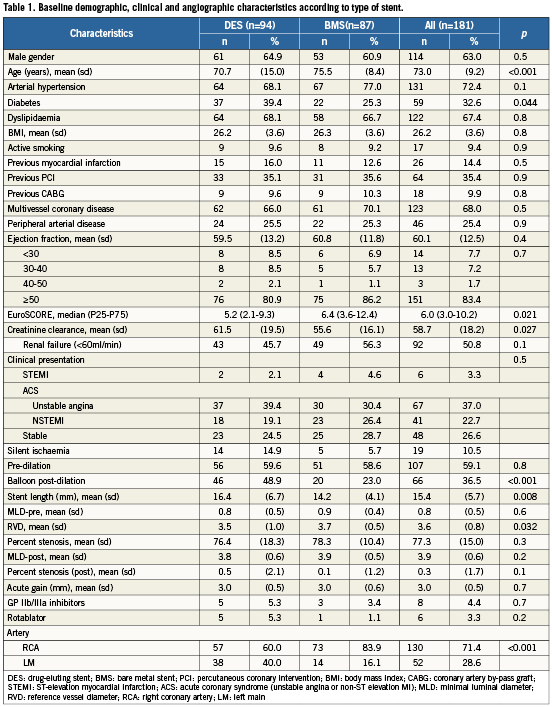
Compared with BMS-treated patients, DES-treated patients were younger, had a higher proportion of diabetics, had a higher proportion of stenting in LM than in RCA, had longer stent length, lower reference vessel diameter, post-dilation with non-compliant balloon was more often used, and a lower median (IQR) logistic EuroSCORE. The radial artery was the preferred approach for most of the interventions (73%). Intra-aortic balloon pumps (IABP) were not inserted routinely. Six patients had rotational atherectomy (all in RCA); intravascular ultrasound (IVUS) was not routinely used to control final result after stent deployment.
In-hospital outcomes
Immediate angiographic success was achieved in all patients. There were two peri-procedural non-Q-wave MI (peri-procedureal complications=1.1%). One was in a patient presenting with unstable angina, undergoing ostial LM stenting with a DES. The procedure was badly tolerated by the patient due to pressure damping from the guiding catheter causing profound hypotension. TIMI 3 flow was achieved but transient inotropic support and IABP placement were necessary. The second was a patient with an almost occlusive ostial RCA lesion. After balloon predilation, slow-flow occurred. Optimal TIMI3 flow was achieved after BMS placement but there was a significant rise in CK-MB, without ECG changes. There were no deaths, CVA, repeat PCI or emergency bypass surgery during hospitalisation.
Follow-up (Table 2)
Median follow-up time was 23.9 months (IQR 12.1-37-7) and was possible in 178 (98.3%) of the entire cohort. Twelve patients (6.7%) underwent routine angiography, 39 (21.9%) patients had repeat coronary angiography during follow-up due to symptoms or silent ischaemia. No ISR was found in the routine angiography group, whereas ISR was documented in 26 (26/39 = 66.7%) of the group that presented due to symptoms or silent ischaemia. Among these, 14 (53.8%) patients presented with unstable angina and 12 (46.1%) had stable angina or silent ischaemia.
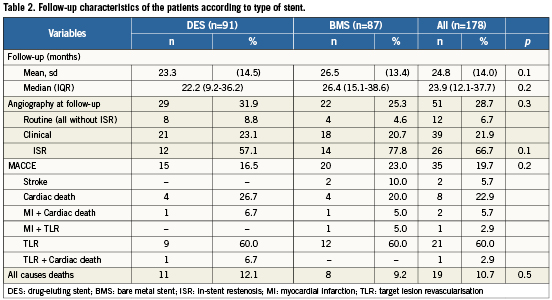
Overall there were 19 (10.7%) deaths: 11 were adjudicated as cardiac and eight as non-cardiac related (cancer: 2; sepsis: 2; chronic respiratory failure: 2; diabetic nephropathy: 1; complications related to non-cardiac surgery: 1). In the “cardiac death” group, seven patients died due to sudden cardiac death, two patients died after an MI, one patient died following CABG for LM restenosis with complications related to surgery and one patient died in cardiogenic shock due to probable stent thrombosis.
MI occurred in three (1.7%) patients, two of them died (previously described) one year after the index procedure, both with multivessel disease unsuitable for revascularisation (localisation of the MI unknown).
One definite (non-target vessel related) and one probable stent thrombosis occurred (one target vessel related probable stent thrombosis for the entire cohort at follow-up=0.6%). The definite case occurred in a patient in whom a BMS had been implanted in the ostial RCA, and one year later a DES to the left anterior descending artery (LAD). The patient presented with an ST-elevation MI due to LAD stent thrombosis caused by premature clopidogrel discontinuation. At angiography, ostial RCA restenosis was documented. PCI was performed to LAD and to treat the RCA restenosis, and the patient recovered uneventfully. The probable case of stent thrombosis occurred in a patient treated with BMS implantation to the LM who presented with cardiogenic shock to a primary care hospital three days after the procedure.
CVA occurred in two patients. One lacunar stroke occurred after PCI to treat a restenosis in a remote vessel. The other patient suffered a stroke one month after index PCI.
ISR was documented in 26 patients, TLR was performed in 23 (23/178=12.9%, DES=10 [5.6%] BMS=13 [7.3%], p=NS), three continued on medical therapy due to diffuse disease of the distal target vessel. Three patients underwent balloon angioplasty only, 17 had repeat stenting and three underwent CABG. Two more patients went on to CABG due to repeated restenosis, therefore a total of five (2.8%) patients underwent cardiac surgery during follow-up. MACCE occurred in 35 (19.7%) patients.
Almost all TLR occurred in RCA-treated patients (22/23). Six cardiac deaths occurred in the LM-treated subgroup; five in the RCA-treated.
At follow-up, differences between BMS and DES-treated patients for MACCE-free and TLR-free survival adjusted for the imbalances between groups in Table 1 were not statistically significant (Figures 1A and 1B).
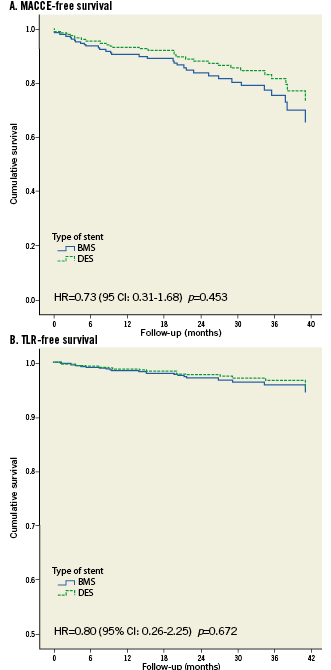
Figure 1. Cox proportional hazards model adjusted for possible confounders for MACCE-free (A) and TLR-free (B) survival between BMS and DES. A. MACCE-free survival. B. TLR-free survival.
Predictors of MACCE, TLR and Cardiac death (Table3)
All cardiac deaths occurred in patients with a logistic EuroSCORE >10%.

By univariate analyses, TLR was significantly associated with the stented artery (RCA vs. LM HR=9.49, 95% CI: 1.28-70.41, p=0.028), BMI (HR=1.12, 95% CI: 1.01-1.25, p=0.032), and stent length (HR=1.07, 95% CI: 1.00-1.13, p=0.038). By multivariate analyses, the predictors for TLR were age (HR=0.96, 95% CI: 0.92-1.00, p=0.039), and the treated artery (RCA vs. LM, HR=10.2, 95% CI: 1.37-75.45, p=0.024). Figure2 illustrates the TLR-free survival according to the type of artery.
Figure 2. Cox proportional hazards model for TLR-free survival according to the type of artery.
MACCE was associated with a logistic EuroSCORE >10% (HR=4.66, 95% CI: 2.38-9.12, p<0.001) and EF <50% (HR=2.15, 95% CI: 1.03-4.49, p=0.041). At multivariate analyses, only logistic EuroSCORE >10% remained as a predictor of MACCE (Figure3).

Figure 3. Cox proportional hazards model for MACCE-free survival according to EuroSCORE>10%.
Discussion
The main findings of our study are: 1) PCI of AOL can be performed with a high rate of success and a low rate of peri-procedural complications; 2) At follow-up, no significant differences were found in outcomes following DES and BMS implantation; 3) High-risk profile (logistic EuroSCORE>10%) is the only independent predictor of MACCE in the long term.
In the DES era, “off-label” PCI accounts for nearly 60% of all procedures, with worse outcomes when compared with “on-label” indications20. In a consecutive, unselected series, like in our cohort, we accept the reported MACCE rate for these complex, off-label indications for PCI. We recognise similarities in study design with previous reports12-15. Although comparisons should be made with caution, our MACCE rate is comparable to one study with consecutive patients15, but is higher than in another14. It may be related to the higher age of our population and higher rates of previous MI. Moreover, the logistic EuroSCORE was >10% in a quarter of our cohort. MI and CVA rates were low, similar to other reports12-15.
We did not find significant differences in MACCE rate between DES or BMS. The advent of DES reduced the restenosis and hence TLR rates when compared with BMS, for a wide spectrum of lesions and patient subsets, with a lesser impact on mortality20,21. While it is logical that coronary vessels with a small diameter cannot accommodate neointimal growth after BMS placement rendering them more suitable for DES22,23, this benefit is more difficult to prove in larger vessels24. Even allowing for the anatomical particularities of AOL described in the introductory section, we believe that the achieved post-procedure MLD of 3.8±0.6mm and 3.9±0.5mm (p=0.2) for DES and BMS, respectively, may be responsible for the results in terms of TLR, and hence in MACCE rate. However, it should be stressed that in the study of Park et al14, follow-up angiography was more than 70% for both types of stent and is a more objective way to assess restenosis and TLR than clinically driven angiography.
The multivariate analyses showed logistic EuroSCORE >10% as the only independent predictor for MACCE. This tells us that it is the patient risk profile, rather than the angiographic characteristics or the type of stent that mostly determine adverse clinical events in the long term.
In our cohort, almost all TLR occurred in RCA-treated patients. A recent paper evaluated differences between RCA and LM AOL by IVUS, concluding that both ostia had the same high frequency of negative remodelling, same morphology, and poor correlation between angiography and IVUS minimal luminal area25. Furthermore, LM ostium had a larger external elastic membrane, while minimal luminal area was smaller for the RCA. These features are similar to another series where restenosis rates were higher for RCA than for LM ostial disease, suggesting that less distensibility and excessive rigidity of the aorto-ostial RCA junction leads to chronic stent recoil26. One could always speculate that patients having ISR in RCA consult more often due to symptoms, whereas LM-treated patients could experience a more malignant event. However, our sample numbers are skewed and underpowered to allow a meaningful conclusion to be reached.
One must not forget that TLR of AOL is probably also influenced by some additional technical factors compared to non AOL. Plaque debulking with rotational atherectomy was only used six times in our series. In everyday practice, low profile balloons and stents allow good immediate and long-term angiographic results for avariety of lesions, reducing the needs for the classical debulking devices as they were used in the past. However, sometimes AOL are very calcified, and plaque modification may be advised to achieve better stent deployment and therefore to reduce TLR. Consequently, under-expansion of the stent may be more frequent for AOL and may explain the lower impact of DES. The systematic use of IVUS for AOL may allow identification of those lesions, which would benefit from rotational atherectomy and also improve optimisation of stent expansion.
PCI of AOL is also technically demanding due to the difficulty in correctly evaluating the position of the ostium by conventional angiography. Misplacement of the stent with incomplete coverage of the ostium could explain some of the TLR independent of the type of stent (BMS or DES) and may explain the lower impact of DES in AOL. The use of multiple projections and some specific techniques for AOL may lower the risk of misplacement of the stent, in an attempt to achieve the delicate balance between complete plaque scaffolding without excessive protrusion into the aorta. One technique is to place the guide catheter close to the balloon stent and in contact with the vessel ostium. Then we pull back the guide catheter and the balloon stent together as a unit, while imaging in at least two projections before inflating. The use of a second wire, curving in the coronary sinus, may be helpful to define the junction of the coronary artery and the aorta, an important landmark for stent placement. There are, however, some alternative techniques that can help to cover the ostium correctly. The Szabo technique27-29 and recently the use of dedicated devices like the Ostial Pro™ (Ostial Solutions, Kalamazoo, MI, USA)30 have shown encouraging results for exact placement of the stent in ostial stenosis when compared with conventional angiographic guided procedures.
Limitations
Some limitations to this study should be addressed: 1) This is a retrospective, single centre study, lacking the well-known advantages of randomised clinical trials; 2) Systematic follow-up angiography was not performed in most of the patients, which could lead to a underestimation of restenosis if the patient is asymptomatic and a non-invasive ischaemia test was not performed; 3) Due to the relatively small sample size, the possibility that our study was underpowered to detect differences in the outcome between DES and BMS cannot be strictly ruled out, but it offers a reasonable estimation of the expected magnitude of the effect for eventual larger studies; 4) MI criteria was still based on rises of CK and CK-MB and not on troponin. Therefore, minor periprocedural infarcts could have been missed.
Conclusions
In our cohort, PCI of AOL can be performed with a very high immediate success and low rate of complications. We did not find different event rates between DES and BMS. At follow-up, EuroSCORE>10% was the only independent predictor for MACCE.
Conflict of interest
The authors have no conflict of interest to declare.

