Abstract
Aims: We report the clinical and angiographic results of the OPEN I study, a multicentre prospective single-arm study evaluating both the drug-eluting and bare metal STENTYS® stents in the treatment of coronary bifurcation lesions.
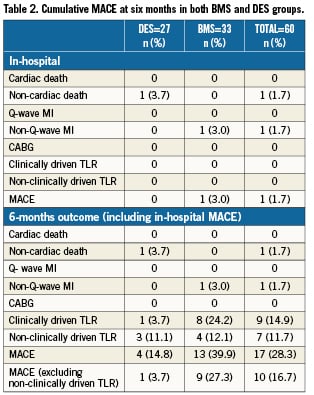
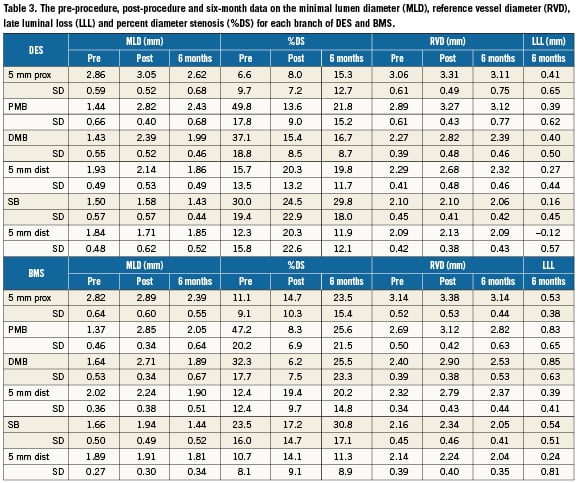
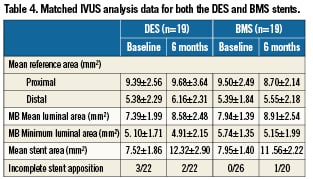
Methods and results: The STENTYS® stent is a provisional, self-expanding, nitinol stent with small interconnections that can be disconnected by balloon angioplasty in between the stent struts to provide access to the side branch (SB) and full ostium coverage. In nine European centres, 60 stents (33 BMS, 27 DES) were implanted in 63 patients (procedural success of 95.2%). Angiographic QCA and IVUS were used to measure acute gain and late loss. The Medina classification showed 35 patients (58%) had disease affecting the SB (true bifurcations) and 19 patients (32%) had disease in all three arms. The average bifurcation angulation pre-stenting was 60°±21°. Post-stenting, disconnection was performed on 90% of the stents implanted. In 18 cases, disconnection was followed by SB stenting with all SB stents successfully implanted. Post-stenting, the bifurcation angle was 51°. The primary clinical endpoint, cumulative MACE at six months, was low for DES (3.7%) but higher for BMS (27.3%) with the latter driven exclusively by clinically-driven TLR rates (3.7% vs. 24.2%). No cardiac deaths were recorded at six months and one patient had a non-Q wave infarct. The secondary angiographic endpoint of late luminal loss (LLL) was measured for both DES (paclitaxel) and BMS stents in the proximal main branch (MB), MB, distal MB as well as the SB. The values for DES were 0.39 mm, 0.42 mm, 0.40 mm and 0.16 mm, respectively. The values for BMS were 0.86 mm, 0.87 mm, 0.85 mm and 0.54 mm, respectively. Observed results using matched IVUS analysis at six months revealed an increase in mean stent area (mm2) for DES from 7.52±1.86 at baseline to 12.32±2.90 at six month follow-up (p <0.001); and for BMS from 7.95±1.40 to 11.56±2.22 (p <0.001), with no decrease in minimum lumen area (MLA) for DES (5.10 to 4.91) and a minimal decrease for BMS (5.74 to 5.15).
Conclusions: This first-in-man (FIM) study on the STENTYS® stent showed excellent procedural success and a relatively low MACE with competitively low LLL in both MB and SB at six months for the DES version and LLL comparable to other BMS for the BMS version. The disconnectable struts offered excellent “cross over” to T- stenting when necessary and the increased gains in stent area over time.
Introduction
Coronary bifurcations interventions have lower procedural success and poorer clinical outcomes. This may be related to the technical challenges associated with the various stent strategies but also due to the complexity of the patients1,2. Although a two-stent approach to bifurcation lesions using drug-eluting stents (DES) suggests that there is no obvious benefit in reducing restenosis and cardiac events it is worth pointing out that in 20-30% of cases a two-stent approach is needed to avoid the loss of the side branch3. We recently reported the acute and 30 day results of the first 40 patients of the OPENI study (STENTYS® Coronary Bifurcation Stent System fOr the PErcutaNeous treatment of de novo lesions in native bifurcated coronary arteries), amulticentre, prospective, single-arm study evaluating the safety and feasibility of the STENTYS® Stent (STENTYS SA, Paris, France)4. This is a provisional, self-expanding, nitinol stent (drug-eluting or bare metal) with small interconnections that can be disconnected by a balloon inflation in between the stent struts to provide access to the side branch and consequently cover the ostium (Figure1). It showed a procedural success of 95.5% with a relatively low major adverse cardiac event (MACE) rate at 30 days (5.1%). We now report on the six-month follow-up results of the full OPEN I study.
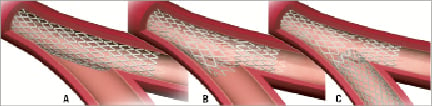
Figure 1. Enhanced provisional technique which provides three possible options: A) main branch stenting only; B) main branch stenting with disconnection; C) main branch stenting with disconnection and T-stenting without a gap.
Methods
Study design
The OPEN I was a prospective, single-arm, multicentre feasibility study in nine European centres to evaluate the safety and feasibility of the STENTYS® coronary stent in de novo bifurcation lesions. Patients were evaluated clinically at one and three months. Quantitative coronary angiography (QCA) and intravascular ultrasound (IVUS) was scheduled at six months. All patients consented and local ethics committee approvals were obtained. All MACE were adjudicated by an independent Critical Event Committee. An independent contract organization (Medpass, Paris, France) monitored the study sites and a core lab (Cardialysis, Rotterdam, The Netherlands) conducted the analysis of the angiographic and IVUS images.
The STENTYS stent
The STENTYS® coronary stent with its unique, self-expanding, Z-shaped nitinol mesh linked by small interconnections enables easy provisional stenting into the side branch (SB). Disconnection of the stent connectors can be accomplished by inflating a standard angioplasty balloon between the stent struts. This is possible all around and along the stent, except for the two most proximal and distal rows5. This is unlike some other dedicated bifurcation stents where positioning and access to the SB can be challenging and cumbersome. The stent is available in two forms, bare metal and paclitaxel drug-eluting (0.8μg/mm2 of stent) incorporated in ProTeqtor® (Hemoteq AG, Würselen, Germany), a durable polymer matrix of polysulfone (PSU) and soluble polyvinylpyrrolidone (PVP) that acts as an excipient. The standard stent length available in the trial was 23mm with a target proximal vessel diameter of 3.5mm to 4.5mm. It could be maximally expanded up to 7.0mm diameter at the carina, a typical size observed for these bifurcations. The entire device was contained within a 7Fr guide catheter-compatible, rapid-exchange delivery system that utilised a single, standard 0.014”guidewire.
Patient population
Patients were enrolled if they had any objective evidence of ischaemia in the territory of the bifurcation lesion. In addition, the bifurcation had to comply with a diameter of 3.5 to 4.5mm in the proximal MB (>3.0mm in the distal MB) and 2.5mm to 3.0mm in the SB, as well as having a bifurcation angle of between 30° and 70° and the ability to be covered with one stent. The lesion lengths were typically ≤15mm with stenoses >50% and <99%. The main exclusion criteria were left main coronary disease, AMI, presence of intraluminal thrombus and EF <40%. Six centres evaluated both the bare and DES versions, while three centres exclusively implanted the bare metal version. If deemed necessary, a work-horse drug-eluting or bare metal stent was deployed in the SB. All patients were required to be clinically followed for four years. The antiplatelet regimen consisted of aspirin combined with clopidogrel and/or ticlopidine per institutional standards of practice.
Procedural strategy
The procedure is performed in a 7Fr guide catheter. Both branches of the bifurcation are wired with standard 0.014” guidewires and the MB appropriately pre-dilated. The STENTYS self-expanding stent is then deployed in the main branch across the bifurcation by retracting the covering sheath, and subsequently the delivery system is withdrawn. The guidewire from the main branch (or a secondary wire) is then pulled back and passed through the stent cell closest to the carina. The previously jailed SB wire is then withdrawn and re-inserted into the MB. A standard angioplasty balloon is passed through the ostium of the SB and inflated to disconnect the stent, thus creating an opening through the SB. At the discretion of the operator, a standard work horse stent was deployed in the SB. IVUS, pre and post QCA were performed as per protocol.
Study endpoints
Primary endpoints were procedural success (defined as technical and angiographic success in the absence of a major adverse cardiac event (MACE) at hospital discharge), MACE at six months together with vessel patency and late loss at six months. Angiographic success was achieved if there was <30% residual stenosis in the MB and the SB with TIMI3 flow in both vessels post-procedure. MACE included cardiac death, stroke, myocardial infarction (MI), coronary artery bypass graft and target lesion revascularisation (TLR) and target vessel revascularisation (TVR). MI was defined as new abnormal Q-waves in accordance with the Minnesota Code for pathologic Q-wave or if the creatinine kinase (CK) was twice the upper limit of normal and there was a rise in the level of MB iso-enzyme of CK (CK-MB). TLR was defined as a repeat treatment of a lesion located within the index coronary artery segment. TVR was defined as a revascularisation of any segment of the index coronary vessel. Clinical vs. non-clinically driven TLR was defined as follows: 1)If the patient had angina and a diameter stenosis (DS)>50%, then this was classified as clinically-driven TLRif the lesion was present in either the SB or the MB; 2)If the patient had angina and the DS was <50% for both SB and MB, then this was classified as no TLR; and 3)If the patient had no angina and a DS >70% for the SB or the MB, then this was classified as clinically-driven TLR. The secondary endpoints included clinical, safety and angiographic parameters. Clinically, the MACE and angina status were assessed at one month and six months. Safety parameters included major bleeding and major vascular complications. Quantitative coronary angiography (QCA, Figure 2) evaluation was performed using the dedicated CAAS5 bifurcation software (Pie Medical, Maastricht, The Netherlands)6. Intravascular ultrasound (IVUS) of the MB and the SB was mandatory before stent implantation, as well as following implantation after disconnection of the stent, and following SB stenting. IVUS was performed at six-month follow-up. All QCA and IVUS images were evaluated by an independent Corelab (Cardialysis, Rotterdam, The Netherlands).
Figure 2. QCA methodology for bifurcation analysis.
Statistical analysis
Continuous variables were presented as mean±standard deviation and categorical variables were presented as counts and percentages. Paired comparisons between post procedure and follow-up were done by the Wilcoxon signed rank test.
Results
Demographics and lesion characterisation
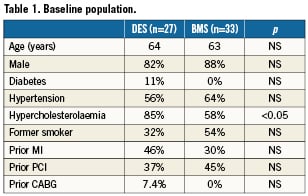
The patient demographics are displayed in Table1. Patients were generally middle-aged men with multiple risk factors for ischaemic heart disease. The only significant risk factor difference between the DES (n=27) and BMS (n=33) groups was hypercholesterolaemia. As previously noted in several studies1,2,7,8, patients with bifurcation lesions are generally more complex and this was apparent from high rates of both previous MI (35%) and previous percutaneous coronary interventions (41%). All patients were admitted on aspirin and were preloaded with clopidogrel.
Lesions classified according to the ACC/AHA classification showed that DES were implanted mostly in type B2 lesions (96%). The majority of BMS were also implanted in B2 lesions (78%) with smaller numbers (18%) in B1 lesions. There was a small portion (4%) of both BMS and DES deployed in patients with typeC lesions. Overall, there was no significant difference (p=0.09) in the choice of stent (either BMS or DES) in the different ACC/AHA lesions. According to the Medina classification for bifurcations (Figure 3), 35 patients (58%) had disease affecting the SB (true bifurcations), and 19 patients had disease affecting all three arms. All patients had disease in the MB with an average lesion length of 11.1±4.7mm. The average bifurcation angulation (between SB and MB) pre-stenting was 60°±21°. This decreased after stent deployment to 51°±14°, remaining unchanged at 6-month follow-up.
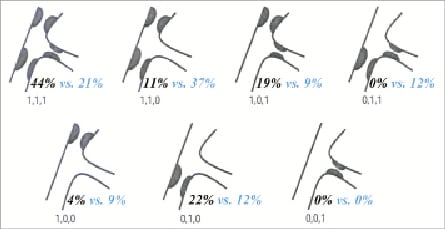
Figure 3. The Medina classification of bifurcation lesions (Black=DES, Blue=BMS).
Primary endpoint: procedural success and MACE
The overall procedural success rate was high at 95.2% comprising 27/29 (93%) for DES and 33/34 (97%) for BMS. The procedure could always be performed as recommended in the procedural section of the protocol with re-wiring possible in all cases and none of the side branches being lost. The three recorded stent failures were attributed to vessel tortuosity and calcification that limited delivery system progression in the vessel. One limitation of the first generation of the device used in this study was that it was only 7Fr guide catheter compatible. One patient had a LM dissection and underwent emergency bypass surgery. Lesions were prepared by pre-dilating the MB with or without the SB in all DES cases and in 97% of BMS procedures. Disconnection was performed in 90% of DES and 92% of BMS as in some cases the stents were placed too distally and this did not allow for the disconnection of the stent. Provisional stenting of the SB was performed in five (18.5%) DES and 13 (39.4%) BMS cases. In the DES group, all SB stents used were paclitaxel-eluting. In the BMS group, five of the SB stents used were BMS (38%) and the other eight were DES (62%) with everolimus (5), biolimus (1) zotarolimus (1) or sirolimus (1). Simultaneous kissing balloons (SKB) were performed in all cases where a disconnection or SB stenting was performed.
The cumulative MACE at six months is given in Table2. AMACE rate of 14.8% was recorded in the DES group and was significantly higher in the BMS group (39.9%, p<0.05). This was primarily driven by the higher clinically-driven TLR rates observed in the BMS group as compared with that noted in the DES group (24.2% vs. 3.7%). MACE rates excluding non-clinically driven TLRs were 1/27 (3.7%) for the DES group and 9/33 (27.3%) for the BMS group. There were no cardiac deaths at six months and only one patient was diagnosed as having a non-Q-wave infarct.
Coronary angiography at six months
Follow-up quantitative coronary angiography (QCA) was performed in 57 cases (32 BMS and 25 DES). In three cases, QCA evaluation was not possible due to vessel overlap. The minimal lumen diameter (MLD) and late luminal loss (LLL) at six months for each branch of DES and BMS are presented in Table 3. The LLL (mm) in the proximal and distal MB for BMS patients were notably higher than that observed in the DES group: in the proximal MB, 0.83mm in BMS vs. 0.39mm in DES; and in the distal MB, 0.85mm in BMS vs. 0.40mm in DES, comparable to LLL traditionally observed with other BMS7 and paclitaxel eluting stents13. This was also the case with the %DS at six month follow-up: in the proximal MB, there was a DS of 23.5% in the BMS group vs. 15.3% in the DES group; and in the distal MB, the DS was 20.2% in the BMS group vs. 19.8% in the DES group. QCA analysis of the DES group demonstrated that when taking into account both clinically and non-clinically driven TLR, only one in-segment restenosis was observed in the MB, which was proximal to the stent. In the BMS group, in-segment restenosis was observed in four (13%) patients proximal to the MB stent, and seven patients (23%) distal to the MB stent. Fewer cases of in-segment restenosis in the side branch were noted when the SB was stented. No in-segment restenosis in the SB was reported in the DES group (average SB lesion length 12.05mm) when the SB was stented (0/5) compared with arestenosis rate of 4/21 (19%) in side branches which were not stented. In the BMS group (average SB lesion length 9.96mm), only 1/13 (8%) SB stents had restenosis as compared to 4/20 (20%) cases when the side branch was not stented. For all patients, when a SB stent was placed, the restenosis rate in the SB was 6%; when no SB stent was placed, the restenosis rate was 20%.
No stent thrombosis was observed. Core laboratory angiographic analysis showed there was one (1.7%) possible moderate stent fracture described as a fracture without clear separation of the stent segment.
Matched intravascular ultrasound (IVUS) at six months
Matched IVUS at six months (including only those patients who had analysable IVUS results post-procedural and at six months) is depicted in Table4 and demonstrates no significant difference in the mean reference area (mm2) in both the proximal and distal segments of both the BMS and DES groups. There was an increase in the mean luminal area (mm2) observed at baseline and at follow-up (DES: 7.39±1.99 vs. 8.58±2.48; p>0.05 and BMS: 7.94±1.39 vs. 8.91±2.54; p>0.05). The minimal luminal area (MLA) was relatively similar at baseline and follow up for DES but there was some decrease for the BMS group. However, there was a marked increase in mean stent area (mm2) from baseline to six months (DES: 7.52±1.86 vs. 12.32±2.90; p <0.0001 and BMS: 7.95±1.40 vs. 11.56±2.22; p<0.0001).
Discussion
The OPEN I trial was designed to show the safety and feasibility of a self-expanding stent with unique disconnectable struts which avoid the problems of wire wrap, stent positioning and other device complexities commonly encountered when treating coronary bifurcations. The trial was initiated primarily using BMS and this was later changed to the exclusive use of the paclitaxel eluting stent. The six-month data for the STENTYS® stent demonstrated effectiveness in all Medina classifications in a 30-70° range of bifurcating angulations with excellent procedural success (95.2%). The mechanism of deployment is now shown to be effective. The simple disconnection of the stent struts enabled standard workhorse stents (four in the DES group and 13 in the BMS group) to be easily tracked, positioned and deployed in the SB allowing a provisional T-stenting approach, if necessary3,5. As expected, the MACE in the DES group was lower than in the BMS group (14.8% vs. 39.9%) due to the higher clinically-driven TLR observed in the BMS group (24.2%). Historical data on other BMS used in bifurcations has demonstrated comparable TLR rates to the STENTYS BMS and ranged from 16% to 39%7. Early data on the use of sirolimus-eluting stents in bifurcation lesions suggested a higher restenosis rate after T-stenting possibly related to incomplete coverage of the side branch ostium8. However, this is addressed by the unique property of the STENTYS engineered struts that can be dislocated to offer complete carina coverage, as demonstrated by OCT post fenestration in one centre that performed OCT measurements (Figure 4).
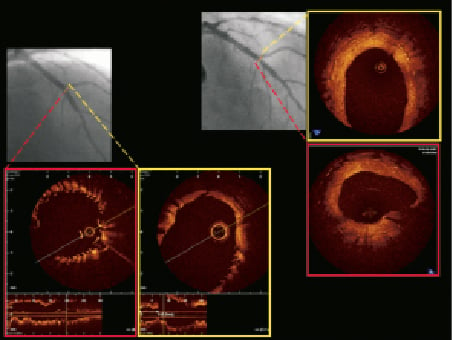
Figure 4. OCT pictures of main branch (red) and side branch (yellow) post-procedure (left) and at six months (right) showing apposition both in the distal main branch and the ostium of the side branch. Complete strut coverage and the wide open ostium of the side branch is shown at follow-up.
Angiographic follow-up performed in 95% of patients demonstrated much lower LLL in the target main vessel and side branch (0.42±0.45mm and 0.16±0.45mm, respectively) in patients treated with the DES. A single case of angiographic in-segment restenosis was noted with the DES due to a lesion proximal to the main vessel stent (TLR 3.3%). All four angiographic SB restenoses observed with DES occurred in cases where the side branches were not stented. Similarly in the BMS group, only 1/13 (8%) of the stented side branches had angiographic restenosis at six months, compared to 4/18 (22%) restenoses when no SB stent was deployed. This suggests that excellent results can be attained with a “cross-over” two-stent approach.
The optimal stenting strategy in managing bifurcations is contentious9. Several large trials such as the Nordic Bifurcation Study, CACTUS and BBC1 have shown equivalent MACE rates for one- and two-stent strategies, ranging from 3.4 to 19%. Similar findings have also been seen in a recent meta-analysis10-13. Moreover, in these trials the two-stent strategies had increased periprocedural myocardial infarction and higher rates of restenosis and stent thrombosis. It was also noted in the CACTUS trial that although there were equivalent outcomes after crush-stenting and the provisional approach, one-third of the patients in the one-stent/provisional approach arm had to cross over to the two-stent arm due to either a residual stenosis in the side branch (72% of cases), poor flow (1.9%) or a significant dissection in the side branch (39%). Astent that covers the carina during a provisional approach could be best suited to avoid these problems and this is a benefit seen with the STENTYS stent, which can also be easily converted to use a two-stent T-stenting technique. Recent fractional flow reserve (FFR) data suggests that it is important to recognise that fenestration into the branch after stenting of the main branch should not be solely based on angiographic appearance14. This is because a shift in the carina angle after placement of the stent often creates an illusion of significant ‘pinching’ of the ostium. Even in cases that angiographically had >75% stenosis at the ostium of the side branch, follow-up at nine months on reopening jailed side branches did not influence MACE, advocating that stents directed to managing side branches first may not necessarily be indicated.
The observed SB late loss of 0.16mm in the DES group and 0.54mm in the BMS group is lower or comparable to those previously reported using only DES (0.34-0.53mm)15-17. The low SB %DS (30% and 31% in the DES and BMS groups, respectively) is also comparable with other studies in bifurcations with DES (24-31% in the Nordic study). IVUS matched DES and BMS data of the MB showed marginal increased mean luminal area, but a significant increase in mean stent area (7.52mm2 increased to 12.32mm2 and 7.95mm2 increased to 11.56mm2) at six months. This confirms that the stent is able to conform to the vascular remodelling which occurs following deployment due to changes in flow dynamics. This could potentially eliminate the occurrence of late acquired stent malapposition (Figure 4)18.
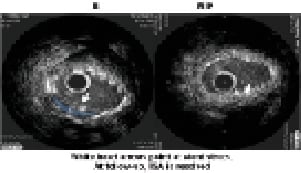
Figure 5. IVUS analysis showing resolution of in-stent malapposition at six months.
Limitations
This was a first-in-man (FIM) study in a small number of patients using DES and BMS stents. The design was non-randomised and operator bias could have influenced the decision to provisionally stent the SB. Although the study results give a clear indication of the feasibility and short-term safety of the stent, longer follow-up data and randomised study designs will lead to more definite conclusions.
Conclusions
This FIM study on the STENTYS® stent demonstrated an excellent procedural success of 95.2%. There was a relatively low MACE in the DES version that also had a competitively low LLL in both the MB and the SB at six months. The precision-engineered disconnectable struts offered excellent “cross over” results for T-stenting and the increased gains in stent area over time are in line with the properties of a self-expanding stent that can limit the occurrence of late stent malapposition.
Conflict of interest statement
R.Spaargaren is an employee of STENTYS. The other authors declare no conflicts of interest.

