Abstract
Transcatheter aortic valve implantation (TAVI) has developed rapidly in the past few years and is expected to expand further in the near future. A reasonable number of large multicentre registries and randomised clinical trials have been performed, which have provided a consiaderable quality of evidence necessary to ensure optimal use and optimal patient outcomes. Currently, a large number of different valves have been approved in Europe, with a varying amount of supporting evidence, which complicates the process of valve type selection. This article reviews the evolution and fundamental aspects of prosthesis type selection in patients undergoing TAVI, and summarises the most relevant clinical studies in this context.
Introduction
In 2002, transcatheter aortic valve implantation (TAVI) was performed for the first time in a human by Alain Cribier1. The valve used was constructed of bovine pericardium sewn inside a balloon-expandable (BE) stainless steel stent1,2. A few years later, Grube et al reported the first-in-human TAVI with a self-expandable (SE) device3. Subsequently, technological refinements of both BE and SE prostheses were reported, and successful implantation was repeatedly documented in observational registries2,4-6. On the basis of registry data, the SE CoreValve (Medtronic Inc., Minneapolis, MN, USA) received CE mark approval for transfemoral implantation in 2007, followed by the BE Edwards SAPIEN transcatheter heart valve (Edwards Lifesciences, Irvine, CA, USA) in 2008.
Selection of TAVI prostheses: the early days
Soon after commercialisation, the majority of European centres started their TAVI programme with either one of the two devices. The SE CoreValve (porcine pericardial tissue, long self-expanding nitinol frame) was available in 18 Fr and in 26 and 29 mm sizes, while the BE Edwards SAPIEN valve (bovine pericardial tissue, short stainless steel frame) was available in 22-24 Fr and in 23 and 26 mm sizes. Consequently, in the limited number of centres using both technologies at that time, device selection was largely based on the size of the iliofemoral arteries (favouring the SE device in small-sized vessels), and the size of the native aortic annulus (favouring the SE device in large anatomies). Notably, valve sizing was largely based on two-dimensional measurements obtained from echocardiography. In a study reporting TAVI outcomes from a single centre offering all possible combinations for transcatheter treatment using both devices available at that time and three types of access (transapical for the BE device, transsubclavian for the SE device, and transfemoral for both), the procedural success rate was 92.7%7. The authors emphasised that the availability of two different devices, delivered via three different approaches, had made the TAVI procedure increasingly feasible for a wider range of high-risk patients with severe aortic stenosis.
A few years later (in 2010), the newer-generation BE Edwards SAPIEN XT valve (Edwards Lifesciences) received CE mark approval. This modified version with a cobalt-chromium frame offered a reduced sheath size of 18-20 Fr for the transfemoral route. Shortly thereafter, a full size matrix of prostheses became available for both BE and SE devices. Consequently, the number of centres utilising both technologies steadily increased, and the number of patients treatable with either device increased in parallel. In addition, the recognition of the complex anatomical nature of the aortic valve, annulus and root has sparked a paradigm shift in pre-procedural imaging, moving towards three-dimensional valve sizing largely based on multislice computed tomography (MSCT), which substantially reduced procedural complications such as paravalvular leaks with both devices8,9. Selection of TAVI prostheses was then strongly dependent both on operators’ familiarity with the device and on certain anatomical and clinical factors that were thought to favour one device over the other. For instance, patients with an impaired left ventricular function were commonly treated with the SE valve to avoid the prolonged periods of rapid ventricular pacing required for the implantation of the BE valve.
Registry data on balloon- and self-expandable valves
Subsequently, a number of multicentre registries reported on the comparative short-term performance of both prostheses10-16. A summary of these registry data is shown in Table 1. In brief, major clinical outcome measures such as mortality and stroke were comparable with both technologies. The need for a new permanent pacemaker was consistently higher with the SE device, which could be explained by the interaction of its long nitinol frame with the left ventricular septum.
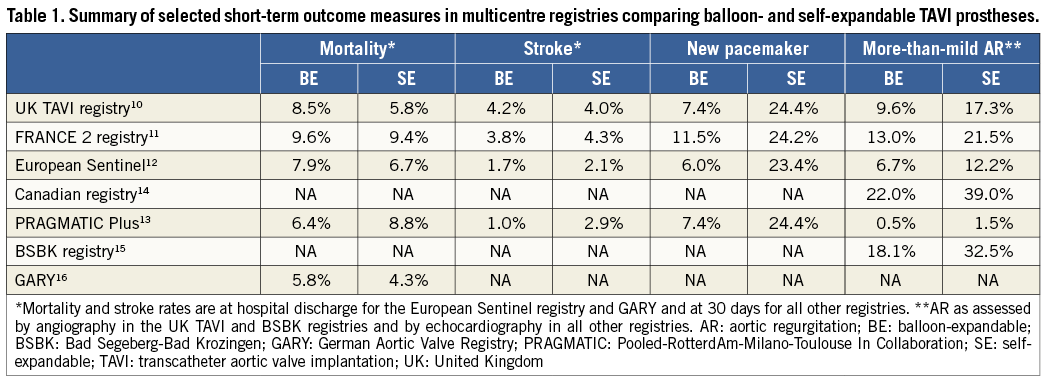
Another finding frequently observed in the majority of these registries was the higher rate of residual more-than-mild aortic regurgitation (AR) with the SE device. The reported incidence ranged from 1.5 to 39% for the SE device and 0.5 to 22% for the BE device. A similar observation was reported in a meta-analysis conducted by Athappan and colleagues, where the incidence of more-than-mild AR was 16.0% with the SE CoreValve device compared to 9.1% with the BE Edwards SAPIEN valve (p=0.005)17. However, as registry results are naturally hampered by bias and confounding factors, these differences were interpreted with caution. In addition, improvements in pre-procedural imaging and device size selection, refinements in implantation technique, and the recognition of paravalvular leaks as a relevant clinical complication18-20 were important developments with a potential impact on the functional outcome of both valves. Importantly, most of these registries have reported on AR using site-reported echocardiography or angiography, and core laboratory adjudication was only performed in the Canadian study14.
On the other hand, rare but life-threatening complications such as annular rupture or acute coronary obstruction seemed to be higher with the BE valve21, especially in patients with adverse root features such as extensive subannular calcification and with extreme prosthesis oversizing22.
The randomised US trials
Regulatory approval in the United States has lagged considerably behind Europe, as the results of randomised clinical trials were awaited. The Placement of AoRTic TraNscathetER Valves (PARTNER) trials were the first prospective randomised landmark studies, which compared TAVI using the earlier-generation BE Edwards SAPIEN device with medical management in inoperable patients (PARTNER Trial Cohort IB)23,24, and with surgical aortic valve replacement in patients considered to be at high surgical risk (PARTNER Trial Cohort IA)20,25. Based on the results of both trials, the United States Food and Drug Administration (FDA) approved the BE Edwards SAPIEN device for inoperable patients in November 2011, and for high-risk operable candidates in October 2012.
For the SE CoreValve device, the results of two analogous studies were recently reported26,27. The non-randomised CoreValve US Pivotal Trial extreme risk study compared TAVI with the SE CoreValve to a pre-specified objective performance goal in inoperable patients, while the randomised CoreValve US Pivotal Trial high risk study compared TAVI using the same device with surgical aortic valve replacement in patients at high risk for surgery. Based on the results of both studies, the FDA approved the SE CoreValve device for inoperable patients in January 2014, and for high-risk operable patients in June 2014.
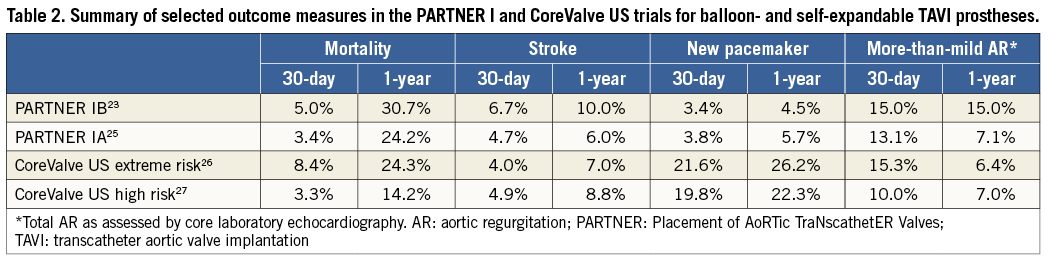
Following the publication of the CoreValve US trials, device comparisons were again performed utilising the reported outcome measures from both the PARTNER and the CoreValve US trials. The fact that the superiority of TAVI compared to surgical valve replacement was only demonstrated with the SE device and not with the BE device sparked a great deal of discussion. A summary of the most relevant findings from the four published studies is provided in Table 2. Notably, and contrary to the European and Canadian registry data, residual AR seemed to be comparable with both devices, with even a slightly lower incidence with the SE device (especially at one year), suggesting an improvement in paravalvular AR over time with the SE device. It is important to mention here that sizing in the PARTENR I trial was based on two-dimensional echocardiography as opposed to three-dimensional MSCT in the CoreValve US trial. In addition, the obligatory presence of an on-site proctor in the CoreValve US trial may have positively influenced the reported outcome. Finally, inter-trial comparisons remain difficult to perform and, in a rapidly advancing field such as TAVI, continuous improvements in patient selection, imaging and implantation techniques render comparisons of trials performed a few years apart extremely problematic.
The CHOICE trial
To date, the Comparison of Transcatheter Heart Valves in High Risk Patients With Severe Aortic Stenosis: Medtronic CoreValve vs. Edwards SAPIEN XT (CHOICE) trial28 is the only randomised clinical trial comparing BE and SE valves, albeit only for the transfemoral route. The trial partially fills the void of randomised device comparisons described above. CHOICE included 241 patients at five German centres experienced in implanting either valve. The design of the trial is summarised in Figure 1. The primary endpoint of the trial was device success, which is a composite endpoint comprising four components: successful vascular access and deployment of the device and retrieval of the delivery system; correct position of the device; intended performance of the heart valve without moderate or severe regurgitation; and only one valve implanted in the proper anatomical location. The correlation of device success to one-year survival has recently been described15. In CHOICE, implantation of a BE valve resulted in a higher device success rate than the SE valve, with device success in 116 of 121 patients (95.9%) in the BE valve group, and 93 of 120 patients (77.5%) in the SE valve group (relative risk [RR] 1.24, 95% CI: 1.12-1.37, p<0.001). This was attributed to a significantly lower frequency of residual more-than-mild aortic regurgitation (4.1% vs. 18.3%, RR 0.23, 95% CI: 0.09-0.58, p<0.001) and the less frequent need for implanting more than one valve (0.8% vs. 5.8%, p=0.03) in the BE valve group. Placement of a new permanent pacemaker was also less frequent in the BE valve group (17.3% vs. 37.6%, p=0.001). With the study being relatively small, there was no difference in 30-day mortality rates (4.1% in the BE valve group and 5.1% in the SE valve group).
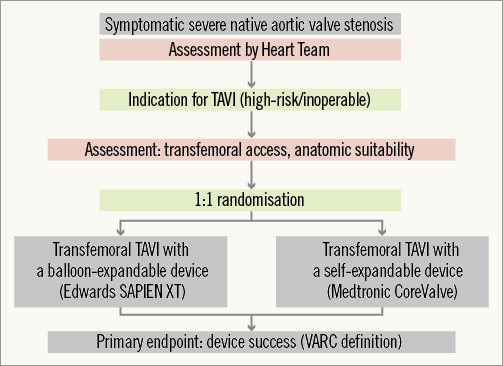
Figure 1. Design of the CHOICE trial28. TAVI: transcatheter aortic valve implantation; VARC: Valve Academic Research Consortium In the CHOICE trial, methods to quantify AR were carefully selected, and included core laboratory angiographic assessment with predefined methods, haemodynamic assessment, echocardiography and magnetic resonance imaging (MRI)28. Choosing angiography as the primary tool for AR assessment was partly based on the better correlations observed with angiography than with echocardiography when both were compared to quantitative cardiac MRI29. Notably, the differences in AR between BE and SE valves were observed despite adequate annulus sizing using three-dimensional annular measurements and proper high deployment of the SE valve. Furthermore, and contrary to popular belief, the performance of the SE valve was particularly poor in patients with large anatomies and those with heavily calcified aortic valve leaflets (Figure 2), which may be related to the suboptimal radial force of the nitinol stent frame. The more frequent need for a second valve in the SE valve group was in line with previous registry data11-13, and supports the notion that newer-generation repositionable valves will be particularly helpful for the SE technology.
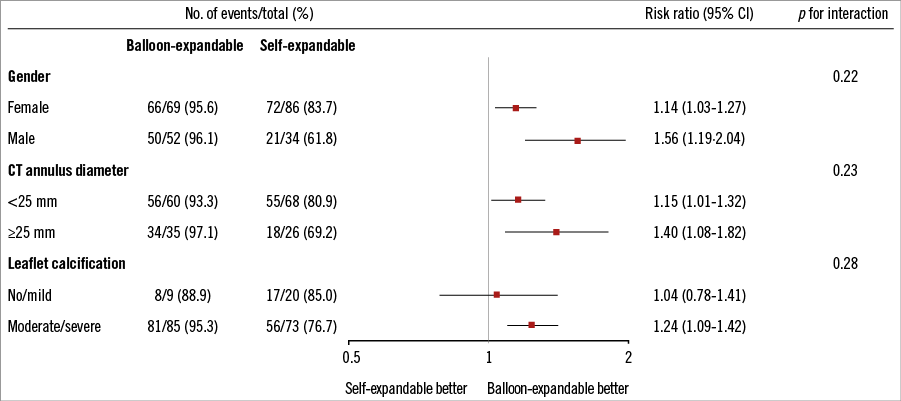
Figure 2. Selected subgroup analyses for device success from the CHOICE trial. Selected subgroup analyses are shown for the primary endpoint of device success among patients who were randomly assigned to undergo transfemoral TAVI with either a balloon-expandable or a self-expandable prosthesis. The p-value for interaction represents the likelihood of interaction between the variable and the relative treatment effect. Odds ratios are for the balloon-expandable vs. self-expandable device28. CT: computed tomography The differences in device success between both devices seemed to affect how patients felt after the procedure. The functional class improvement (94% for BE vs. 87% for SE), readmission rates for heart failure (0% for BE vs. 4.3% for SE), and the quality-of-life score (71/100 for BE vs. 66/100 for SE) at 30 days were slightly better for the BE valve compared with the SE valve. However, mortality rates at short-term follow-up were comparable, while long-term follow-up data are still pending. Therefore, whether the observed differences in an endpoint such as device success will translate into a long-term clinical superiority of one device over the other is unknown and remains to be determined, especially as paravalvular leaks in SE devices may improve over time, as has been recently suggested in the CoreValve US trial26,27.
On the other hand, mean transvalvular gradient was slightly but significantly higher in the BE valve group (8.9 mmHg vs. 6.6 mmHg, p<0.001), and minor stroke and coronary occlusion were numerically (but not statistically) higher. In addition, patient subsets not studied in this trial where SE valves may have benefit include valve-in-valve for small degenerated bioprosthetic aortic valves (less residual gradients due to supra-annular valve function)30, and patients with minimal calcification of the aortic valve with predominant regurgitation31. These and other points may be critical in deciding which valve to use in a given patient.
Selection of TAVI prostheses: where do we stand now?
While prosthesis selection was mainly dictated by the dimensions and specifications of the delivery sheath, the delivery catheter and the anatomical limitations of the available prosthesis sizes in the early days, in contemporary clinical practice, operators are mainly focusing on prosthesis specifications such as frame height, design, radial force and anatomical aortic annulus and root characteristics. A reasonable number of large multicentre registries and randomised clinical trials performed in the last few years have provided a considerable quality of evidence necessary to ensure optimal use and optimal patient outcomes after TAVI. The totality of evidence –at least for the transfemoral route– shows that BE valves allow precise device positioning and have a predictable short-term outcome in a wide range of patients and anatomies, with fewer paravalvular leaks and less need for permanent pacing. However, device iterations should aim at improved haemodynamics and optimising stroke rates. In this context, the new-generation BE SAPIEN 3 valve (Edwards Lifesciences) holds some promise.
On the other hand, an ideal self-expandable TAVI device should be completely repositionable/retrievable to allow accurate device positioning. Device iterations should aim at optimising radial force to provide a better seal in large anatomies and extensive calcification, but should probably maintain the advantage of a supra-annular leaflet function, which is associated with excellent haemodynamics.
With the tremendous amount and pace of progress with TAVI, ten different valves have now been approved in Europe, which makes the process of valve choice even more complicated. While the fundamental characteristics of the valves remain unchanged, newer technologies such as mechanically expanding valves (Lotus; Boston Scientific Corporation, Natick, MA, USA) and non-metallic devices (Direct Flow Medical®; Direct Flow Medical Inc., Santa Rosa, CA, USA) have entered the field and are searching for their indications. Despite their theoretical advantages, all of these “newcomers” are supported by a modest amount of scientific evidence. In addition, procedure-related factors, such as access route, balloon pre-dilatation and periprocedural imaging, sometimes remain controversial.
The majority of TAVI patients will probably be treatable with one valve type. Nevertheless, an expanded device armamentarium is necessary to ensure treatment of the complete spectrum of patients considered for TAVI. In addition, because operator experience is an important factor in ultimate success, local expertise has to be considered when selecting the valve type. The results of the CHOICE trial are important, but currently cannot be interpreted as a surrogate for long-term outcomes, and the question of device durability is still unanswered. Long-term follow-up data and additional rigorous randomised studies are necessary to optimise further selection and use of the currently available devices.
Conflict of interest statement
M. Abdel-Wahab and G. Richardt report receiving an institutional research grant from Medtronic and lecture fees from Boston Scientific. G. Richardt reports receiving lecture fees from Edwards Lifesciences.

