Abstract
Aims: When patients choose percutaneous coronary intervention (PCI) over coronary artery bypass grafting (CABG), they accept an increased long-term risk of repeat revascularisation in exchange for short-term morbidity benefits. This paper quantifies the risk-benefit trade-off faced by patients with multiple vessel coronary artery disease.
Methods and results: Data from the Arterial Revascularisation Therapies Study are used to generate risk-benefit acceptability curves for PCI versus CABG. Risks are measured by the long-term likelihood of repeat revascularisation while benefits are measured by short-term reductions in pain or improvements in health-related quality of life (HRQL). PCI patients faced a risk of 0.81 additional revascularisation events over three years in exchange for being pain-free at one month. A patient would need to be willing to tolerate a risk of 1.06 additional revascularisation events at three years, in exchange for being pain free at one month to be 95% confident that choosing PCI over CABG is risk-effective for him/her.
Conclusions: The risk-benefit framework outlined in this study provides information to enable physicians to help their patients weigh directly each procedure’s risks and benefits. While trade-offs are typically measured in quality-adjusted life years, using pain reduction to reflect benefits may provide a more tangible framework for patients.
Introduction
The optimal mechanical revascularisation technique for multivessel coronary disease (MVD) remains contentious, with advocates supporting both percutaneous coronary intervention with insertion of stents (PCI) and coronary artery bypass grafting (CABG). Patients choose a procedure after consultation with physicians and may weigh a number of considerations in making their decision. Trends in the United States and elsewhere show that the volume of percutaneous procedures has been increasing while CABG rates decline.1,2 These data indicate a growing preference for PCI over CABG for many patients and their physicians, especially for persons age 65 and older.3
Traditional cost-effectiveness analysis is useful for determining the societal benefit of these procedures, and this avenue of research has been incorporated in a number of comparisons of PCI and CABG.4,5 However, for patients with low cost sharing requirements for both procedures, the choice may be based on the valuation of benefits and risks rather than financial consideration. Clinical trial evidence indicates that both CABG and PCI increase health-related quality of life (HRQL) for patients with MVD. In the long-run, however, CABG shows substantially lower rates of revascularisation when compared with bare-metal stenting (PCI).6 Receiving these deferred benefits of reduced rates of repeat revascularisation requires accepting higher morbidity such as delayed relief from pain or improvement in HRQL in the time period immediately following the procedure.
Economists have become interested in using probabilistic methods to portray the trade-off between risks and benefits as well as the implicit valuation that patients make when one technique is chosen over a competitor. For example, probabilistic simulation modelling has been used to estimate the joint density of therapeutic risks and benefits from two pharmacological prophylactic treatments for deep-vein thrombosis.7 In this study, we use clinical data from the Arterial Revascularisation Therapy Study (ARTS) to estimate the trade-off between the benefit of quick reductions in post-procedure pain and HRQL when choosing PCI versus the increased risk of repeat revascularisation. Specifically, this analysis uses a risk-benefit acceptability curve (RBAC), which is analogous to the cost-effectiveness acceptability curves used to portray uncertainty in the economic evaluation of health care technologies.8 The RBAC provides estimates of the probability that a particular approach such as PCI is viewed by the consumer as being risk-effective (i.e., having greater value of benefits than cost of risks, analogous to cost-effectiveness) over different preference thresholds for the risk-benefit trade-offs identified. The estimates provided may be useful to physicians in explaining trade-offs between risks and benefits of alternative treatments to their patients, and the methodology can be applied to the current generation of PCI versus CABG studies as more complete data become available.
Methods
Between April 1997 and June 1998, ARTS randomised 1,205 patients with MVD to receive PCI with bare-metal stent implantation (n=600) or CABG (n=605).5 Key details of the original study relevant for this analysis are the inclusion/exclusion criteria and the data collection strategy. The indications for revascularisation for enrolment in ARTS included silent ischaemia, stable or unstable angina pectoris, and the presence of at least two de novo lesions located in different major epicardial coronary arteries. Exclusion criteria included left ventricular ejection fraction <30%, left main stenosis, history of cerebrovascular accident (CVA), transmural myocardial infarction (MI) within the preceding week, severe hepatic or renal disease, and need for concomitant major surgery.9 All patients gave written, informed consent. Follow-up information was obtained for patients at 1, 6, 12, and 36 months post-randomisation, and the EuroQol EQ-5D instrument was administered to patients at each time point. More PCI patients completed the EQ-5D at one month than did CABG patients (93.5% versus 88.8%, p = 0.005); by six months, the difference was not significant and remained so for the rest of the follow-up period (90.2% vs. 87.8%, p=0.22). All other data completion rates were comparable between treatment groups.
A binary variable indicating the presence or absence of clinical events was used to represent the risk in the decision to undergo PCI. Risk variables were calculated for three outcomes at 12 and 36 months post-randomisation: (1) additional mechanical revascularisation after the randomised therapy; (2) additional PCI; and (3) additional CABG. Separate analysis of death, CVA and MI are not provided because their incidence did not differ in the ARTS trial, though disagreement remains about whether these outcomes differ between the two procedures. We used three measures to quantify the quality-of-life benefits of PCI. The pain/discomfort question on the EQ-5D was recoded to a binary variable indicating absence or presence of pain at the time of interview. For a more comprehensive view, a preference-weighted composite health-related quality of life measure (HRQL) was calculated from the EQ-5D survey responses.10 Patients who did not complete the EQ-5D because they died prior to its administration were assumed to have a HRQL of zero for all subsequent time points. The HRQL measure was also used to estimate quality-adjusted life years (QALYs) post-intervention; since HRQL was only measured at four points post-randomisation, we approximated QALYs using a linear area under the curve approximation (except for the first month, where the patient’s HRQL at one month post-randomisation was used to calculate the entire first month of QALY since PCI offers such immediate pain relief relative to CABG).
Incremental risk was calculated as the difference in the proportions of patients experiencing additional revascularisation for patients assigned to PCI versus CABG. Similarly, incremental benefit was calculated as the difference in proportions of patients reporting being free from pain and the difference in mean HRQL scores. Incremental risk-benefit ratios (IRBR) for various follow-up points in time were calculated by dividing incremental risk by incremental benefit:
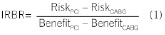
Bootstrapping with 5,000 replicates was conducted to form risk-benefit acceptability curves (RBAC) for each combination of selected risk time points (12 and 36 months) and one month benefit.8 A longer time frame (up to three years) was chosen for risks while a shorter time frame was chosen for benefits because differences in benefits (pain or HRQL) were concentrated in the first few months after initial revascularisation (Figures 1A and 1B).
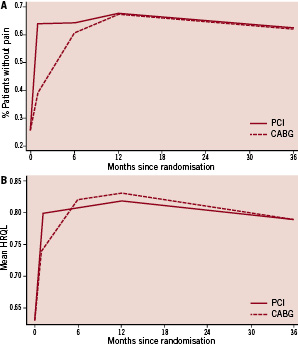
Figure 1. A. Proportion of patients free from pain after randomisation. B. Mean HRQL scores obtained by value weighting EQ-5D results.
All inferential statistics were conducted at a two-tailed alpha level of 0.05, and results were analysed on an intention to treat basis. Data analysis was conducted in SAS version 9.1.3 (SAS Institute, Cary, NC, USA) and Stata version 10.1 (StataCorp LP, College Station, TX, USA).
Rather than attempt to explicitly discount pain or clinical events benefits, we compared the long-term risks of PCI with its short-term benefits. As discussed later, discounting would increase the relative value of PCI because of greater short term pain reduction benefits in contrast to the benefits from CABG that take longer to accrue.
Results
As previously reported, rates of revascularisation were higher among patients randomised to PCI than CABG, with the incremental risk for PCI versus CABG increasing from 17.2% to 20.2% when moving from one to three years post-randomisation (Table 1).6,11
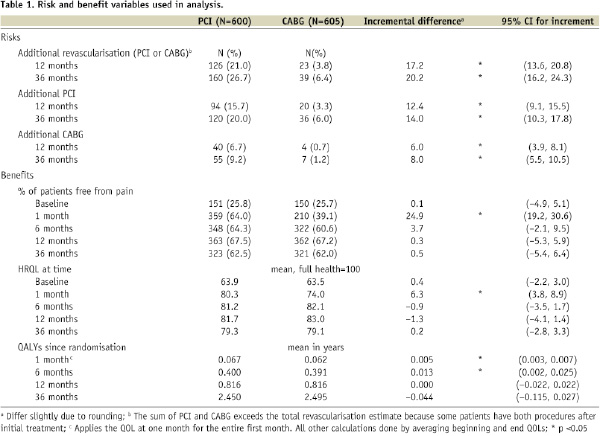
At three years post-randomisation, of the 160 PCI patients who had experienced additional revascularisation 15 (2.5% of trial arm) had experienced both an additional PCI and an additional CABG while of the 39 CABG patients with repeat revascularisations only two (0.3%) had experienced both procedures after their initial treatment.
PCI patients were more likely to be free from pain and had higher HRQL scores shortly after randomisation, though the effects were no longer statistically significant by six months after intervention. Table 1 shows that the incremental benefit of PCI decreases (from 0.25 to 0.04 on the freedom from pain measure and from 6.3 to –0.9 on the HRQL measure) when moving from the one month to six month time point, as many CABG patients recovered from the immediate post-procedure pain and HRQL impairment that occurs during the first few months following CABG. Figures 1A and 1B illustrate this trend graphically and show that composite HRQL from CABG reaches that of PCI slightly earlier than does freedom from pain, with both measures statistically equal from both procedures by 12 months post-randomisation. When QALYs are calculated using the HRQL scores, the difference in QALYs is minimal and not statistically significant after six months. Since repeat revascularisations accrue over time but significant differences in benefits occur only at one month after initial revascularisation, we focus our comparisons on the trade-off of long-term risk for short-term benefit.
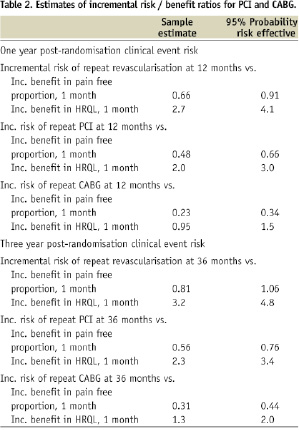
Together, the increased risk of revascularisation from PCI and benefit trends for both procedures lead to increasing risk-benefit ratios as time from initial treatment randomisation increases (Table 2). Bootstrapped estimates of the incremental risks and benefits and the distribution of the incremental risk/benefit ratio illustrate the trade-off more completely. All of the sampled incremental risk and benefit estimates were positive, meaning there was always a trade-off between PCI and CABG and the IRBRs were always positive. As shown in the last column of Table 2 (bottom half), our data indicate that a patient or his/her physician would need to be willing to tolerate a risk of 1.06 additional revascularisations, including risk of 0.76 additional repeat PCI events and 0.44 additional CABG events, at three years in exchange for being pain free at one month in order to be 95% confident that choosing PCI over CABG is risk-effective for him/her. (By risk-effective, we mean that the risk is worth the benefit.) Similarly, for each one percentage point improvement in HRQL at one month post-randomisation, a patient would need to be willing to tolerate 4.8 additional revascularisations to be 95% confident that they are making a risk-effective choice in choosing PCI.
Figure 2 demonstrates the information in Table 2 visually through the formation of the risk-benefit acceptability curves (RBACs) from the distributions of incremental risk-benefit ratios.
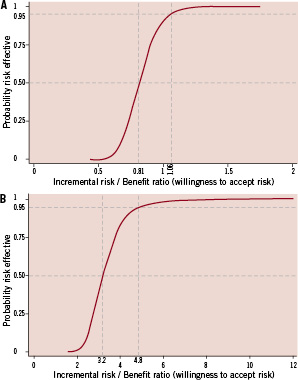
Figure 2. A. Risk benefit acceptability curve for incremental risk of additional revascularisation within three years versus freedom from pain at one month. B. Risk benefit acceptability curve for incremental risk of additional revascularisation within three years of randomisation versus HRQL benefits at one month.
The RBAC illustrates the probability that PCI is risk-effective given a particular risk-benefit ratio threshold.12 Each RBAC in Figure 2 represents the cumulative density of a single ratio of risk and benefit variables (the proportion of sampled ratios less than or equal to the specified ratio). Consequently, movements along the curve from the left to the right represent a willingness to accept a higher level of risk of revascularisation from PCI in return for the immediate relief (i.e., reduction in pain or improvement in HRQL) from PCI relative to CABG. Discounting of benefits and risks (not done for simplicity of presentation) would shift the curves more to the left since the pain reduction from CABG is delayed relative to PCI.
We noted earlier that more PCI patients completed the EQ-5D at one month than did CABG patients. The lower response rates for the EQ-5D at one month post-randomisation among CABG patients likely leads to an underestimation of the pain reduction benefits of PCI, as patients with high pain at one month were probably less likely to complete surveys. To assess this possible bias, we recalculated the risk benefit ratios under the assumption that the difference in response rate between CABG and PCI patients at one month after randomisation (4.7 percent) was comprised entirely of patients in pain (the least favourable case for CABG). Our point estimates of the risk benefit ratios would in this case overestimate the ratio by only 7.4%, indicating that any bias from differential response rates for CABG and PCI patients at one month is not substantial.
Discussion
This report uses risk-benefit ratio estimation and the corresponding risk-benefit acceptability curves to model the trade-off between PCI and CABG. We model the choice of PCI over CABG as that of accepting greater long-term risk of additional revascularisation in exchange for reduced post-procedure morbidity, specifically pain and composite HRQL. Rather than discount risks or benefits, we explicitly compared short-term benefits and long-term risks by comparing benefits at one and six months with risks at 12 and 36 months. In order for patients or their physicians to be 95 percent confident that their decision is risk-effective, a patient choosing PCI over CABG would need to be willing to accept the risk of 1.06 additional revascularisations over the next three years per patient benefiting by being free from pain at one month post-randomisation. Analogously, a patient would need to accept 4.8 additional procedures per percentage point increase in HRQL.
Although the trial data are one decade old, the objective of this analysis is to develop a methodology that can enable more informed decision making on the part of patients and their physicians in choosing between PCI and CABG when neither procedure has a compelling indication or contraindication. Individual patients will value the trade-off differently; for some, exchanging the increased risk of repeat PCI or CABG to obtain short-term pain relief and HRQL increases will be acceptable, while others may prefer to endure short-term pain to obtain a higher probability of avoiding a subsequent revascularisation. Additionally, patients may prefer to risk undergoing multiple PCI procedures rather than a single CABG, or they may prefer to avoid the risk of requiring CABG subsequent to PCI and instead have CABG initially.
The interpretation of RBACs has generally followed Bayesian guidelines. This approach has advantages from a policymaker’s perspective, because it allows for the determination of whether an intervention is risk effective given a specified tolerance for risk. This delineation has clear value in exercises such as determining how much risk of severe adverse events would have to be tolerated to receive benefits. Composite HRQL measures generated from the EQ-5D provide a more comprehensive view of a patient’s status than outcomes based strictly on freedom from pain. Yet the interpretation of HRQL is less intuitive, especially for patients. We suggest that the freedom of pain measure, while a simplification of HRQL, provides a useful way for physicians to work with patients to compare these two procedures, as presence and absence of pain is intuitive for most patients and providers. Distributions of risk-benefit ratios can provide patients and physicians with a sense of the trade-off between risks and benefits in direct comparison, not simply the separate magnitudes of risks and benefits. Patients would be best informed by being given both the risk-benefit ratio and the size of the absolute risks and benefits. The changes at the margin are likely most relevant for policy makers, and the fact that the statistically significant differences in outcome occur for just a short period after initial revascularisation mean that the absolute increase in QALYs is very small.
Our study is not without limitations, especially in terms of applying the results in current clinical practice. Conceptually, our model depends on the assumption that the primary risk a patient experiences when choosing PCI over CABG is that of repeat revascularisation, and the primary benefit is decreased pain. While most clinicians accept those risks and benefits as correct, especially with the use of bare-metal stents, assertions about differences in rates of death and more severe complications are more contentious.13-16 Furthermore, drug-eluting stents have reduced risk of restenosis compared with that of bare-metal stents, although controversy remains about the magnitude of this effect and potential increased risk of thrombotic events,17-19 though longer-term follow-up data are not yet available. The results of the SYNTAX and FREEDOM trials, among others, will explore in detail the different risks and benefits of PCI with drug-eluting stent insertion when compared with CABG in patients with MVD. One year data from SYNTAX indicate the incremental risk of any repeat revascularisation was reduced to 7.6%, compared with 17.2% in ARTS, although differences in inclusion criteria preclude direct comparison.20
An additional important consideration is the fact that the heterogeneity of treatment effects within populations is always of concern when applying trial results to clinical practice.21,22 For patients with less extensive disease or who have a shorter life expectancy, PCI may be more appealing, with the opposite being true for patients in better general health with more extensive cardiac disease. As described above in the study methods, ARTS only included patients with double and triple vessel disease (predominantly double) and excluded patients with a number of medical complications. The risk-benefit acceptability curves presented in this analysis are consequently most applicable to relatively healthy patients with MVD without several other medical complications, a relatively small portion of the universe of CAD patients. Yet the potential value of the approach for improving information available for decision-making by physicians and patients remains. Ultimately, as more comprehensive data are available, risk-benefit determination can become increasingly personalised.
Acknowledgements
This analysis was conducted at The University of North Carolina at Chapel Hill, NC, USA. This study was supported by grants from the National Institute of General Medical Sciences (5T32-GM008719 for JF) and the National Institute on Aging (1R01-AG025801 for SS). Suzanne West provided early inspiration for the analysis.

