Abstract
Aims: Arterial access selection is crucial during transcatheter aortic valve implantation. When traditional femoral access has been deemed unfeasible the left subclavian artery has been used successfully. In cases where even the latter was ineligible, we opted, despite the lack of any data, for the right subclavian approach. We hereby present the results of the first series available. Our aim was to evaluate the feasibility and performance of the CoreValve ReValving System (CRS) implantation via the right subclavian artery in patients with contraindication to femoral and left subclavian accesses.
Methods and results: Among 300 patients who have undergone CRS implantation, 70 (23%) have been treated via the subclavian approach, 10 via the right subclavian artery and 60 via the left. Demographic features were quite similar except for the presence of significant left subclavian disease in all patients treated via the right subclavian artery. The success rate was 100% for both groups. At 30-day follow-up, there was no significant difference in terms of all-cause mortality and cardiac mortality between right vs. left subclavian approach (0% vs. 6.6% and 0% vs. 6.6%, respectively). Consistent results were observed at a mean follow-up of 12±7.9 months (all-cause mortality: 10% vs. 15%). Incidences of new AV block requiring PM implantation were also statistically equivalent.
Conclusions: CRS implantation via the right subclavian artery was as feasible and safe as the left subclavian approach. It poses very particular technical issues but should be considered when more conventional approaches are inadequate in order to provide patients with a further chance to be treated effectively.
Introduction
Transcatheter aortic valve implantation (TAVI) is now widely accepted as a valid alternative to the traditional surgical approach in those patients deemed at high or prohibitive surgical risk for morbidity and mortality1,2. Moreover, the concept of a “heart team” in charge of the patient from the initial clinical evaluation to the follow-up is receiving increasing support.
Among a number of aspects inherent to a patient’s management, the selection of the appropriate access is crucial in order to reduce complications. Besides the transfemoral, other accesses such as the left subclavian3, the transaortic, and the transapical for the Edwards SAPIEN prosthesis (Edwards Lifesciences, Irvine, CA, USA), have been used successfully4.
Of note, the right trans-subclavian approach has been suggested as feasible for the CoreValve ReValving System (CRS)-TAVI but, to the best of our knowledge, no data have been published concerning procedural and clinical outcome.
We hereby present the results of patients treated with CRS-TAVI in the two Italian centres using this approach.
Methods
A “heart team” with a cardiologist, an interventional cardiologist, a cardiac surgeon and an anaesthesiologist evaluated the patients, judging the level of risk for surgical aortic valve replacement and therefore the expected rate of complications as well as morbidity and mortality. Although limited in their capacity to predict for TAVI, logistic EuroSCORE as well as STS score were also calculated in order to obtain a numeric and objective assessment of the procedural risk. We retrospectively collected and analysed the baseline, procedural and follow-up data of patients treated via the subclavian approaches in our centres.
TECHNIQUE OF CRS-TAVI
The CoreValve bioprosthesis is a trileaflet porcine pericardial tissue valve, sewed inside a self-expanding nitinol frame. The current third-generation CoreValve ReValving System (Medtronic, Inc., Minneapolis, MN, USA) was used in all patients. The femoral approach, either surgical or percutaneous, was always used when feasible.
In case of unfavourable iliofemoral anatomy (excessive tortuosity), unfavourable aortic anatomy (excessive tortuosity, aneurysmatic dilation with thrombus), or extensive atherosclerotic disease, both subclavian approaches were assessed angiographically and by means of angio-CT scan (Figure 1). In the absence of severe tortuosity and/or atherosclerotic disease, the left subclavian artery was chosen as it theoretically allowed a more favourable orientation of the CoreValve delivery system compared to the right subclavian; however, where needed, the right subclavian approach was used. The presence of a patent left internal mammary artery bridging the left anterior descending coronary artery was not a contraindication for the left subclavian approach, provided that there was a subclavian artery diameter of at least 7 mm.
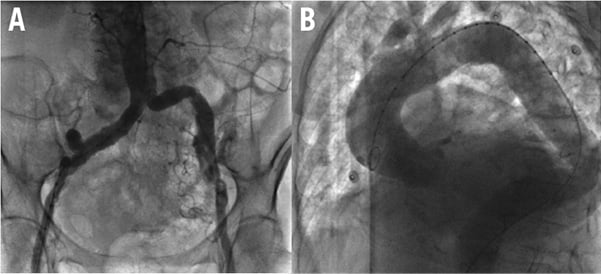
Figure 1. Examples of conditions contraindicating the femoral approach. A) diffuse, bilateral atherosclerotic disease of the iliofemoral arteries. B) excessive tortuosity of the abdominal and thoracic aorta.
In all cases, the subclavian artery was isolated by a cardiac surgeon as described elsewhere3. After direct puncture, an 18 Fr sheath, the same as that used for the femoral approach, was advanced retrogradely into the aortic arch and ascending aorta, underneath the brachiocephalic artery.
The type and intensity of anaesthesia were chosen on a clinical basis. Balloon valvuloplasty was performed under rapid pacing (160 to 180 bpm) before CoreValve deployment. Aspirin (100 mg/d) was administered before the procedure and continued indefinitely. All patients also received clopidogrel (300 mg loading dose), followed by 75 mg daily for three months, unless a prolonged dual antiplatelet therapy was indicated for a pre-existing condition. During the intervention, the patient received weight-adjusted intravenous heparin to achieve an activated clotting time of 200 to 250 seconds for the duration of the procedure.
A temporary pacemaker was left “in place” for at least 24 hours post procedure and then removed, in the absence of atrioventricular blocks.
DEFINITIONS AND FOLLOW-UP PLAN
Endpoints were defined according to VARC definitions5. Clinical follow-up by way of office visits was carried out at one and six months. The rate of safety endpoints was evaluated at 30 days, and that of efficacy endpoints at six months.
STATISTICAL ANALYSIS
Categorical variables were expressed as a number and percentage of patients. Continuous parameters were expressed as mean ±standard deviation. Differences between treatment groups were assessed by the Fisher exact test or χ2 test for categorical variables and by Student’s t-test for continuous variables, as appropriate. Statistical tests were performed with IBM® SPSS® Statistics, version 17 (SPSS Inc., Chicago, IL, USA). A two-tailed value of p<0.05 was considered statistically significant.
Results
STUDY POPULATION
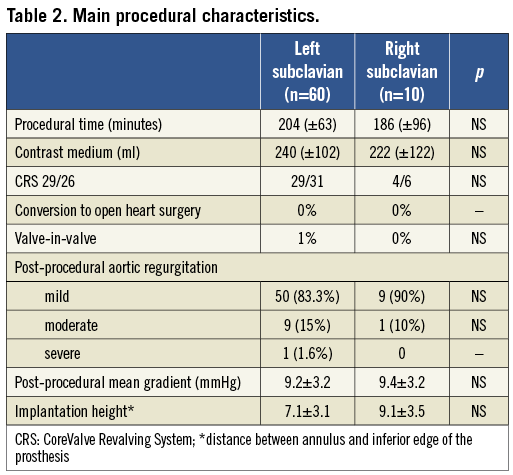
Among an overall population of 300 patients treated with CRS, in 70 cases (23%) subclavian arteries were chosen, 60 left (85.7%) and 10 right (14.3%). Table 1 reports differential features of patients treated via the left or right subclavian artery. The reasons that led to the choice of the right subclavian approach were (Figure 2): a) presence of significant atherosclerotic disease of the left subclavian artery (five patients); b) previous percutaneous treatment (two patients); c) excessive tortuosity of the left subclavian artery (two patients); and/or d) small calibre of the left subclavian artery (calibre <7 mm) with a patent left internal mammary artery bridging the left anterior descending coronary artery (one patient).
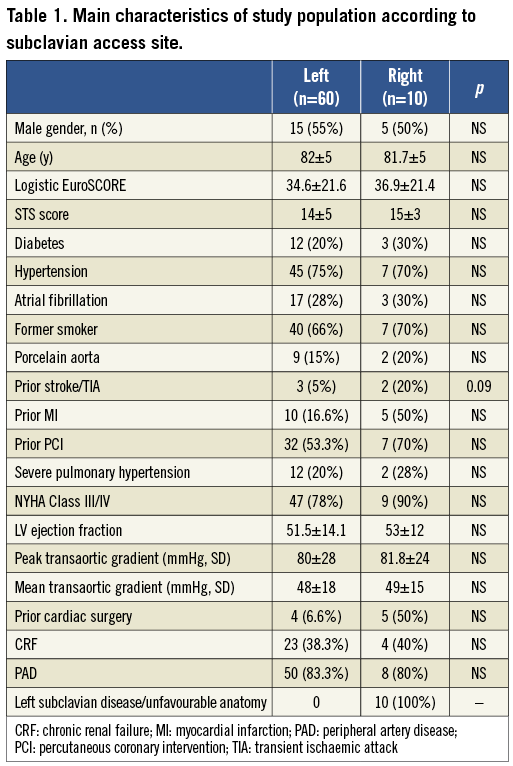
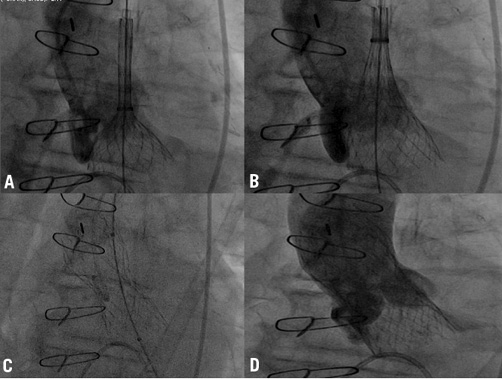
Figure 2. Conditions contraindicating left subclavian access. A) shows the presence of a previously implanted stent. B) shows an ulcerated plaque to the proximal segment of the left subclavian artery. C) shows a case of excessive tortuosity of the left subclavian artery. D) shows a case of small left subclavian artery (calibre <7 mm) with a patent left internal mammary artery bridging the left anterior descending coronary artery.
Other clinical characteristics were substantially equivalent between the two groups. The logistic EuroSCORE was very high in both groups, reflecting the expected very high surgical risk that was mainly driven by diffuse significant atherosclerotic disease.
PROCEDURAL AND DEVICE SUCCESS
Device success according to VARC definitions was 100% in both groups. From a procedural point of view (Table 2), use of the right subclavian approach was not associated with a longer procedural time or with a larger contrast volume. Moreover, the acute prosthesis performance when implanted via the right subclavian was satisfactory in all but one case in which a moderate aortic regurgitation was observed, perhaps treated with balloon post-dilation. The occurrence of new AV block requiring permanent pacemaker implantation did not significantly differ between the two groups.
SAFETY ENDPOINTS
The rate of safety endpoints was quite low at 30 days (Table 3) and not statistically different between the two groups, thus essentially showing the overall safety of the right subclavian approach (all-cause mortality and cardiac mortality between right vs. left subclavian approach were 0% vs. 6.6% and 0% vs. 6.6%, respectively).
EFFICACY ENDPOINTS
At a mean follow-up (Table 4) of 12.4±7.9 months, CRS implantation performed well in both groups, achieving an overall mortality of 15% in the left subclavian group (8.9% excluding the fatalities within the first month) and 10% in the right subclavian group.
Discussion
TAVI has obtained progressive diffusion worldwide as a consequence of encouraging and consistent results across several national registries6-9.
In the context of the entire process that goes from the first diagnosis through the clinical evaluation by the “heart team” to the procedure itself and follow-up, the selection and management of arterial accesses pose specific challenges3,10,11.
CRS implantation via the left subclavian artery showed itself to be as safe and effective as implantation via the more traditional femoral approach3.
Unfortunately, there are cases in which both femoral and left subclavian approaches are not feasible as a consequence of significant atherosclerosis and/or unfavourable anatomy.
The benefit of TAVI in respect of medical therapy is well known. Thus, in order to provide the patients with the chance to be treated more effectively, we opted for the right subclavian approach despite the absence of any reference literature concerning this “off-label” indication. Some encouraging results have been published concerning a small population of patients treated via the “transaortic right mini-thoracotomy” approach12; however, we have chosen the less invasive approach, although a direct comparison has never been done.
The results we present here confirm a substantial equivalence in terms of both safety and efficacy with respect to the left subclavian approach, thus suggesting that the right subclavian approach should be considered where other approaches are unsuitable.
Nevertheless, some technical issues need to be considered before advocating the implementation of this approach over traditional ones.
As for the surgical approach, the right subclavian approach requires much attention to the right common carotid artery (CCA). In particular, the 18 Fr sheath must be positioned without impairing the right carotid flow. The procedure has actually been performed while keeping the sheath distant from the aorta most of the time, in order to check for the CCA flow. The sheath was pushed towards the aortic valve plane only when needed to support the CRS positioning, in order to limit the impairment of the CCA flow as much as possible. This is different from what is done from the left subclavian artery, where the sheath is positioned into the ascending aorta and then adjusted, usually pulled back, during the deployment of the CRS. This manoeuvre is obviously facilitated by the use of a short sheath. At present there are some dedicated sheaths, such as the E-asy plus® (JOTEC GmbH, Hechingen, Germany) which is available with a length of 20 cm and a hydrophilic coating.
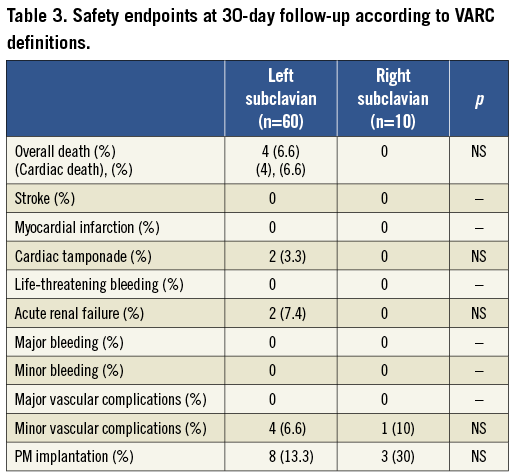
Moreover, considering the very short distance between the tip of the sheath and the aortic plane and in some cases the presence of severe tortuosity, it is also crucial to use a very stiff and supportive wire in order to advance the device safely without pushing against the aortic wall, and to retrieve the knob. To overcome these conditions, for instance, we have successfully used the Lunderquist® Extra Stiff wire (Cook Medical Inc., Bloomington, IN, USA). During the deployment phase, especially when the ascending aorta is horizontal and thus the aorto-ventricular angle is flat, the relative position of the device with respect to the aortic plane looks significantly different (Figure 3), as the device does not follow the natural curve of the ascending aorta which the femoral or left subclavian approaches do. During the first steps, CRS appears excessively vertical to the aortic plane: in particular, the LAO view (Figure 3, Panel A) shows that the CRS will be quite low to the left cusp but very high to the non-coronary cusp. At two thirds of the deployment (Figure 3, Panel B), the position still appears wrong in the LAO view. Nevertheless, by applying some pressure to the system in order to keep this position steady, a good final result can be achieved (Figure 3, Panel C and D).
Figure 3. Deployment of CoreValve. Panel A: initial phase, in LAO view, showing the peculiar position of the device with respect to the aortic valve plane. Panel B: two thirds of the deployment, in LAO view, with orientation of the device still being quite vertical to the aortic valve plane. Panel C: final position of the device in LAO view. Panel D: final position in RAO view.
A major issue relates to the angle between an horizontal line and the aortic plane, i.e., when the latter is >30°, the proper alignment of the CRS appears quite complicated. Patients with such an unfavourable angle (Figure 3) were treated anyway: they were not suitable for other approaches and at the time of this registry there was no experience in transaortic procedures. Thus, a compassionate use was approved by the local “heart team” in order to give patients a better chance to be treated effectively.
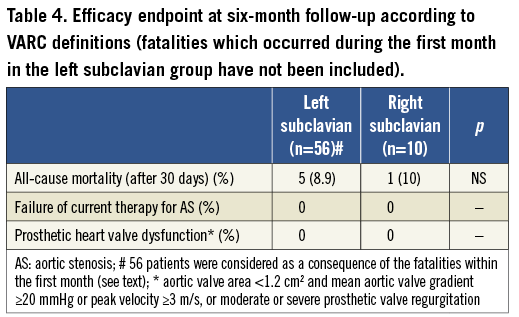
A possible advantage of the right subclavian approach over the left consists in the possibility of retrieving a partially deployed CRS in a relatively safe manner, as the sheath is quite close and straight compared to the tortuous navigation from the aortic valve plane to the left subclavian. Indeed, in one case of left subclavian approach, we were forced to release the valve right at the left subclavian ostium and pass through the frame with another CRS (Figure 4).
Figure 4. Case of complicated left subclavian approach. Panel A shows that the retrieval of a partially deployed CoreValve into the sheath was not feasible. Panel B shows the dilation of the frame with a balloon in order to navigate a new CoreValve to the aortic valve (Panel C). Panel D shows the final result, which was successful with a good immediate performance of the device.
Study limitations
The CoreValve Revalving System implantation, as with any other new technology or treatment, requires a definite learning curve as well as subsequent robust experience in order to reach the highest level of safety and efficacy. Thus, an alternative arterial approach such as the right subclavian should be considered initially only in high-volume centres, before advocating a routine implementation.
Prosthesis alignment is a major issue requiring large experience in manipulating the device, knowing its reaction to pressure, friction and rotation into the aortic root. The direct transaortic route seems able to overcome this issue but, to the best of our knowledge, head-to-head data objectively comparing these alternative approaches are lacking.
Conclusions
This study shows that, in high-volume centres, the right subclavian approach can be at least as safe and effective as the left subclavian approach which, in turn, has results comparable to the femoral approach. From a technical point of view, these data, although concerning a small series of patients, highlight the feasibility of CRS implantation via the right subclavian artery. From a clinical point of view, availability of a further arterial access increases the chances of providing patients with a therapy of proven efficacy.
Conflict of interest statement
F. Bedogni and A. S. Petronio are TAVI medical proctors for Medtronic. All other authors have no conflicts of interest to declare.

