Abstract
Aims: The index of microvascular resistance (IMR) is a relatively new tool that is used to assess microvascular function during routinely performed left heart catheterisations. In order to establish a reference interval for IMR, we investigated a subset of arrhythmia patients with structurally normal hearts and no or minimal coronary artery disease.
Methods and results: Physiological variables, including IMR, were measured in 20 otherwise healthy patients aged 40-60 years (10 males and 10 females) who had been referred for electrophysiological evaluation of suspected atrioventricular nodal re-entry tachycardia. IMR values were non-normally distributed with a median value of 12.6. We established a reference interval, that would be relevant to 95% of the population, of 7.3 (90% CI: 6.6-8.0) - 27.2 (90% CI: 20.8-33.7), using Box-Cox transformation and the robust Horn method. Spearman’s rank correlation analysis revealed no significant relationship between IMR and several different variables.
Conclusions: A reference interval for IMR was established in a population of patients aged 40-60 years with structurally normal hearts, considered to be representative of the general population. IMR was not related to sex, age or any of the other variables tested, suggesting that this reference range can be applied to the general population.
Introduction
The past shortcomings of coronary angiography in the evaluation of coronary artery disease have led to the development of physiological methods that can be readily applied in catheterisation laboratories1,2. Measurement of the index of microvascular resistance (IMR), which is one of these methods, assesses microvascular function during routinely performed left heart catheterisations with selective coronary angiography. IMR can be measured using commercially available intracoronary guidewires equipped with a pressure transducer and thermistors. An increased IMR is believed to be a surrogate marker of pathophysiological processes in the microvasculature of the heart. This method has been validated in animal studies3-5 and further elucidated in studies on patients with acute myocardial infarction6, stable angina pectoris7 and coronary atheromatosis8, and in heart transplant recipients9-11.
IMR is specific to the microvasculature4, but a reference interval for healthy individuals has not yet been established. For ethical reasons the IMR has not been –and will probably never be– evaluated in healthy volunteers. However, as an approximation, a reference interval can be established using data from patients with minimal structural cardiac pathology. Patients with atrioventricular nodal re-entry tachycardia (AVNRT) usually have normal myocardial function and perfusion and are among the healthiest of patients with heart diseases, and we believe that these patients may constitute a feasible reference population12.
The objective of the present study was to establish a reference interval for IMR and assess the association of this index with age, sex and other background variables in a cohort of patients, referred for the evaluation of presumed AVNRT and who did not have obstructive coronary artery disease, who were considered representative of healthy individuals.
Methods
STUDY POPULATION
After obtaining their written consent to participate, 20 patients aged 40-60 years (10 of each gender) who were referred for electrophysiological evaluation of presumed AVNRT were included in the study during 2011 and 2012. The inclusion criteria were a coronary angiogram showing no obstructive coronary artery disease and a fractional flow reserve (FFR) above the ischaemic threshold (defined as FFR >0.80). Patients with other cardiopulmonary diseases, atrial fibrillation or flutter, uncontrolled endocrine disturbances or significant mental disorder, or who were being treated for arterial hypertension or for other reasons unable to comply with the protocol, were excluded.
On day one all patients received a clinical assessment that included measurement of blood pressure, height and weight, echocardiographic examination and venous blood sampling for laboratory profiling, including lipid profile, creatinine, electrolytes, liver enzymes, glucose, haemoglobin A1c, thyroid function, cardiac troponin T and N-terminal-pro-brain-natriuretic peptide (NT-proBNP). Left heart catheterisation and coronary physiological examinations were performed on day two, and an electrophysiological examination was performed on day three.
The study protocol was approved by the Norwegian Regional Committees for Medical and Health Research Ethics (REC), Department REC South East (Approval code 2010/3356).
ECHOCARDIOGRAPHY
Images of the left ventricle were obtained from the parasternal and apical positions, recording standard parasternal short and long axes as well as apical four-chamber, two-chamber and long-axis imaging planes using a digital echocardiography scanner (Vivid 7™ or E9™; GE Vingmed, Horten, Norway). Conventional greyscale video sequences and tissue Doppler loops were recorded, as were blood flow velocities in the pulmonary vein, mitral valve ring and tip, and left ventricular outflow tract using the Doppler technique. Echocardiographic diameters are presented relative to body surface, as estimated using a standard method13.
HEART CATHETERISATION AND PHYSIOLOGICAL ASSESSMENTS
Left heart catheterisation was performed according to our standard routine: a transradial approach with a 6 Fr arterial sheath and 6 Fr diagnostic and guiding catheters. At the start of the procedure 5,000 U of heparin, 2.5 mg of verapamil and 200 µg of glyceryl trinitrate were administered intra-arterially. A physiological assessment was performed if the coronary angiograms showed no or minimal coronary artery disease. The guidewire (PressureWire™ Certus™; St. Jude Medical, St. Paul, MN, USA) used for pressure and temperature measurements was calibrated outside the body and then advanced through the guiding catheter to the ostium and into one of the major coronary vessels, preferably the left anterior descending (LAD) artery. With the pressure sensor at the tip of the guiding catheter in the ostium of the vessel, the pressure readings of the guiding catheter and pressure wire were equalised. Glyceryl trinitrate (200 µg) was administered at the beginning of the physiological assessment and then readministered as necessary (i.e., in the presence of coronary spasms or if the procedure had taken longer than 30 minutes). The guidewire was advanced 5-10 cm down the coronary artery of interest. Baseline thermodilution curves were obtained by intracoronary injection of room temperature saline in boluses of 3-4 ml at least three times until stable registrations were obtained. The resting mean transit time (Tmnr) of the saline and the proximal (Pa) and distal (Pd) pressures were recorded. After maximal hyperaemia was obtained by delivering intravenous adenosine (140 µg/kg/min) through a cubital vein, room-temperature saline was again injected down the vessel as described above. Hyperaemic mean transit time (Tmnh), Pa and Pd were recorded. FFR was calculated by dividing Pd by Pa (Pd/Pa) and averaged over the three beats with the lowest quotient. Coronary flow reserve (CFR) was calculated by dividing Tmnr by Tmnh, and IMR by dividing Pd by the inverse of the median value of the Tmnh, i.e., IMR=Pd×Tmnh4. Continuous equalisation of pressures was confirmed at the end of the procedure by repositioning the pressure wire in the ostium of the vessel.
BLOOD SAMPLING
Creatinine, lipid status and glycaemic control parameters were analysed by routine laboratory methods from blood samples taken in the morning after an overnight fast. Both NT-proBNP and high-sensitivity cardiac troponin T were determined using electrochemiluminescence immunoassays (proBNP II and Elecsys Troponin T high sensitive, respectively; Roche Diagnostic Ltd., Burgess Hill, West Sussex, UK).
STATISTICAL ANALYSIS
Descriptive statistics are presented as mean (SD), median (25th-75th percentiles) or frequency values. Groups were compared using the Student’s t-test for independent samples or Mann-Whitney U test, as appropriate.
The normality of the distribution of IMR values was evaluated using normal probability plots and the Kolmogorov-Smirnov test (with Lilliefors significance correction). The median of IMR values is presented with the 2.5th and 97.5th percentiles, as calculated by linear interpolation. The association between IMR and age, sex, body mass index, smoking status and other background variables was assessed using Spearman’s rank correlation. The sample was considered too small for multivariate modelling.
We estimated a reference interval for IMR that would include values in 95% of the population, using Box-Cox transformation of the data and a robust method, using Reference Value Advisor v. 1.3 software14,15. This programme identifies and excludes possible outliers, defining suspected outliers as observations either above quartile three plus one and a half times the interquartile range, or below quartile one minus one and a half times the interquartile range. For the reference interval, we established 90% confidence intervals (CI) for the upper and lower limits.
Other statistical analyses were performed with IBM SPSS Statistics version 20 (IBM, Armonk, NY, USA). We used a 5% significance level, using two-sided tests.
Results
In total, 24 patients had heart catheterisation but four were excluded for the following reasons: diabetes mellitus diagnosed at screening (n=1), development of atrial fibrillation during heart catheterisation (n=1), significant coronary atheromatosis (n=1) and diagnosis of atrial flutter (n=1).
Apart from supraventricular tachycardia, the study subjects were healthy and had structurally and functionally normal hearts as assessed by echocardiography, without any signs of left ventricular hypertrophy (i.e., interventricular septum diameter index, left ventricular posterior wall diameter index and relative wall thickness were all within reference values). All had NT-proBNP and troponin T values within normal reference intervals. Apart from one male who had previously been treated for arterial hypertension with an angiotensin II receptor blocker, none of the subjects had a clinical diagnosis of systemic hypertension and none used antihypertensive drugs. All antiarrhythmic medications (beta-blockers, calcium antagonists or flecainide) were halted at least one week prior to the examination. In addition to two females who used hormonal contraception, six subjects were on the following regular medications: thyroid replacement therapy (n=1), beta-2 agonist/steroid inhalation (n=1), both aspirin and a statin (n=1), aspirin (n=1), a statin (n=1) and a proton-pump inhibitor (n=1).
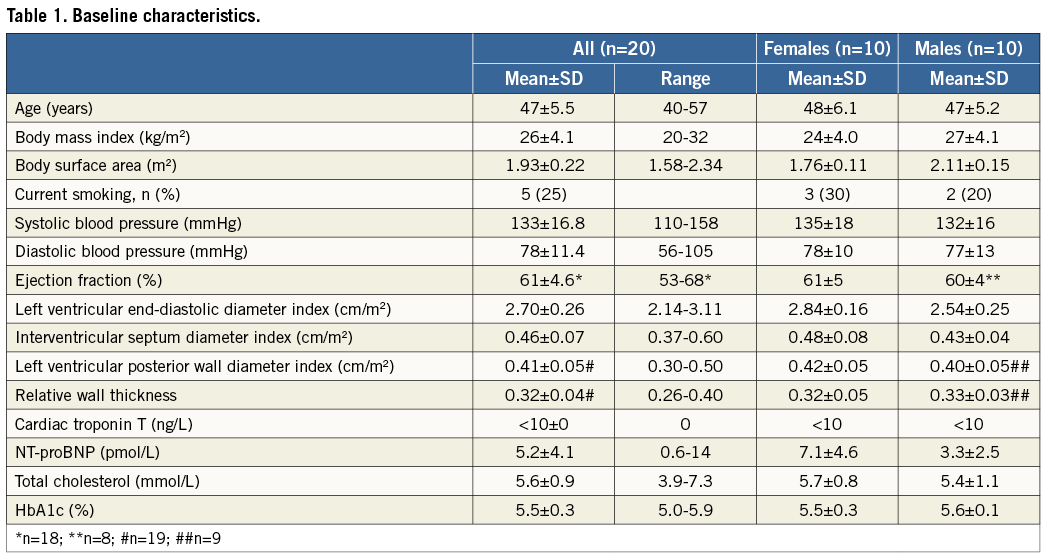
Five subjects were current smokers, and one had stopped smoking two years prior to examination. Baseline characteristics are outlined in Table 1.
Coronary angiography revealed normal epicardial arteries in 18 subjects, while two had slight atheromatosis defined as stenosis severity <33%. FFR was above the ischaemic threshold in all patients, with 75% having a value above 0.90. Physiological measurements were performed in the LAD artery in all except one patient, in whom the circumflex artery was examined for anatomical reasons. Electrophysiological examination confirmed AVNRT in 16 patients, while four were diagnosed with an accessory bundle (atrioventricular re-entry tachycardia [AVRT]) without ventricular pre-excitation (delta waves) on resting ECG. AVRT patients were included in the analysis because inclusion was based on ECG documentation at referral.
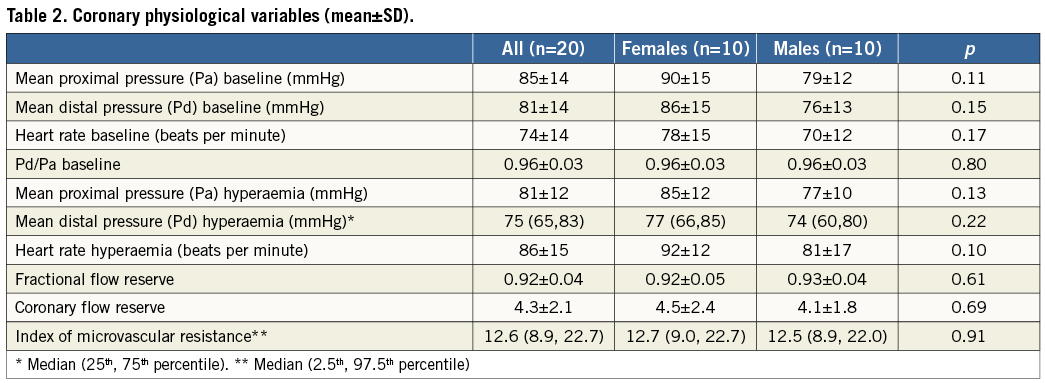
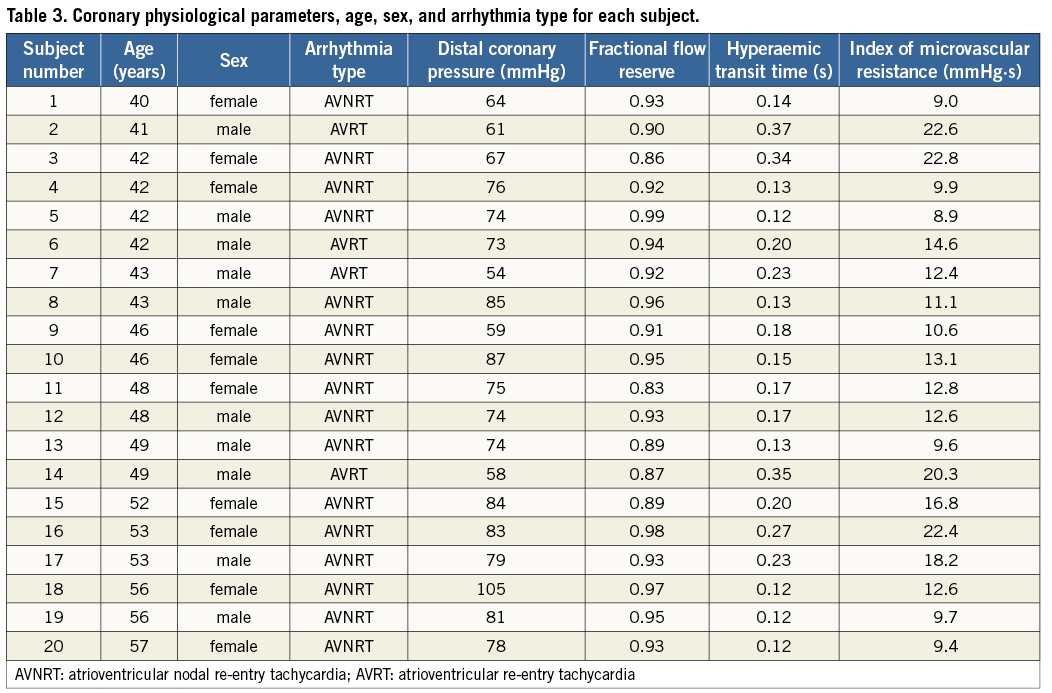
The coronary physiological variables are listed in Table 2. IMR exhibited a non-normal distribution with a median value of 12.6, and 2.5th and 97.5th percentile values of 8.9 and 22.7, respectively. The physiological parameters and details of the age, sex and arrhythmia type for each subject in the study population are given in Table 3.
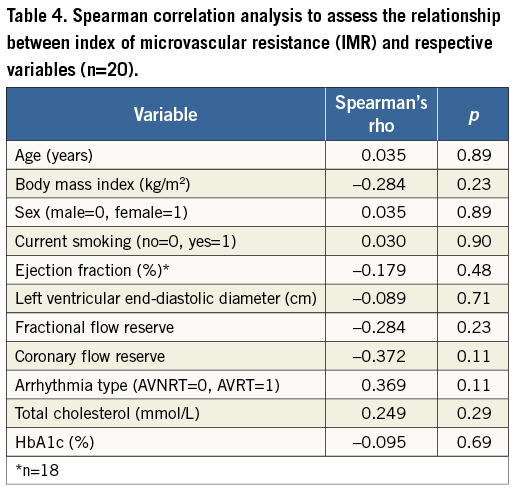
Spearman’s rank correlation revealed no significant relationship between IMR and the patients’ demographics, clinical characteristics, biochemical values and other physiological parameters (Table 4).
Box-Cox transformation of the data and robust estimates was used to establish that a reference interval for IMR that would be relevant to 95% of the population was from 7.3 (90% CI: 6.6-8.0) to 27.2 (90% CI: 20.8-33.7). The total sample was used in the computations since no observations were identified as potential outliers.
Discussion
We assessed the coronary microvascular resistance in a population of individuals with AVNRT/AVRT but who were otherwise healthy, with structurally and physiologically normal cardiovascular functions, as assessed by clinical judgement, biochemical tests, echocardiography and coronary angiography. It might be considered unethical to perform studies for determining IMR in healthy subjects from the general population, and we believe that the population enrolled in the present study is a reasonable substitute. In subjects aged between 40 and 60 years we propose a reference interval for the IMR of 7.3-27.2.
Challenges related to establishing reference intervals are particularly encountered in the field of clinical chemistry. Although at least 40 (and ideally more than 120) individual tests are recommended for establishing reference intervals, this is often not possible. Horn et al published a method for estimating reference intervals for data sets with small numbers of observations15. Four different statistical procedures were compared for samples involving as few as 20 observations. One of the procedures, the “robust Horn interval”, yielded values closest to the true underlying distribution, and we used this procedure to establish the reference interval in the present study. One of the problems with small samples is that outliers could have a greater influence when establishing reference values. However, no outliers were identified in this study, and so the entire sample of 20 observations could be used to establish the reference interval.
Problems regarding cut-off values are also mentioned in echocardiographic guidelines16, emphasising the problem of skewness in the reference population and difficulties in defining clinically relevant limits. Based on the non-normal distribution of IMR values, reporting only the mean (with SD) or median (with interquartile range) will probably yield either an interval that is too narrow or an upper cut-off value that is too low.
There are few previous studies on subjects with insignificant structural heart disease for comparison. Moreover, in contrast to our approach, previous studies have mostly cited mean values, thus further complicating comparisons with the present study. For example, Ng et al17 studied 15 subjects without significant coronary artery stenosis with a mean age of 63 years and found a mean IMR value of 22.6 (SD, 6.0), which differs markedly from the median value of 12.6 in the present study. Their high IMR value may be associated with older age and a higher incidence of risk factors for cardiovascular disease (hypertension, hypercholesterolaemia, diabetes and smoking). Melikian et al studied patients with coronary atheromatosis8 and reported a mean IMR value of 25 (SD, 13). In a control group consisting of patients undergoing left heart catheterisation for other reasons (i.e., seven patients prior to closure of a patent foramen ovale and eight patients prior to electrophysiological examination), they reported a mean IMR of 19 (SD, 5). However, in several of the patients in that study, IMR was measured in more than one coronary artery, and hence all of the measurements were not independent. Overall, in that mixed group of patients with structural heart disease and arrhythmia of unknown aetiology, IMR was higher than in our study.
A recent study involving patients with coronary artery disease demonstrated an association between IMR and both total and low-density lipoprotein cholesterol, and also found that the IMR values in arteries with a stenosis severity of >30% were higher than the IMR values in the present study, with a mean IMR of 26 (SD, 12)18. In total, 68% of their patients were treated with a statin; in contrast, only 10% of the patients (n=2) in the present study were receiving statin therapy, and subjects with coronary artery disease (stenosis severity >33% or FFR ≤0.80) were excluded. No significant association between IMR and total cholesterol was detected in the present study (Table 4).
Another recent study found an IMR-lowering effect of the angiotensin-converting enzyme inhibitor (ACEi) enalapril19. This could not be assessed in the present study since none of the patients used an ACEi.
IMR values are higher in patients with acute coronary syndromes than in those with stable coronary artery disease6,7,20. Studies on IMR in heart transplant recipients report mean values in the range 19-30, depending on time from transplantation10,11; a value of >20 is proposed as a criterion for microvascular dysfunction9.
IMR values in the present study were lower than in the aforementioned studies. This discrepancy may be attributable to differences in the characteristics of the examined populations, such as the age range or the risk or presence of coronary artery or structural heart diseases. The strict inclusion criteria applied in the present study ensured that the included subjects had structurally normal hearts, and hence can be considered representative of the healthy general population. However, the reported reference interval in the present study was wide, which was probably due to variability inherent in the measurement methods and the small size of the sample.
Limitations
IMR was measured in the LAD artery in all except one patient in this study. Whether the reference interval is also valid in the other major coronary arteries was not tested, and this represents a limitation of the study. Clarification of this issue should be sought in future studies. However, a previous study involving patients with coronary artery stenoses found no difference in IMR values between LAD and non-LAD arteries21.
While the angiograms of the included subjects were completely normal except for slight atheromatosis in two patients, the mean FFR value was 0.92 (SD, 0.04). Pijls et al reported an FFR value of 0.98 (SD, 0.02) in a normal LAD artery22, and hence a concomitant minor coronary artery atheromatosis cannot be completely ruled out. The study subjects did not have complaints that would raise suspicion of coronary artery disease, but neither exercise-stress testing nor perfusion imaging was performed to confirm this. The presence of normal arteries could have been further verified using intravascular ultrasound imaging or optical coherence tomography, but neither of these formed part of the study protocol due to ethical considerations. Even if coronary angiograms are normal in patients with AVNRT, elevated troponin levels after arrhythmic episodes are associated with increased risk of death, myocardial infarction and cardiovascular rehospitalisation12. Unfortunately, information on troponin levels during tachycardia was lacking in most of our patients. Furthermore, only patients aged 40-60 years were included, thus limiting the analysis of the association between IMR and age, and so limiting generalisation of the findings beyond this age group.
Maximal coronary hyperaemia was induced by the continuous intravenous infusion of adenosine through an antecubital vein, while the standard method of adenosine infusion in coronary physiological studies is generally via the femoral vein. However, comparisons of infusion routes have shown little difference between femoral and forearm/antecubital infusions23,24.
We kept data obtained from subjects with AVRT in the analysis because inclusion in the study was based on the initial ECG recordings. Furthermore, their baseline characteristics did not differ from those of the AVNRT patients, and there is no indication from information in the literature that they would have had microvascular dysfunction. The sample was on the small side for establishing reference intervals, and would have been even smaller without the subjects with AVRT. However, large samples are not always feasible, and we used a statistical method that was developed for deriving reference ranges for small samples, taking into account the skewness of the distribution14. The relatively small difference between the range of the 2.5th-97.5th percentile calculation and the reference interval based on the robust Horn method supports the feasibility of our method. It is likely that a larger sample would produce a lower upper-reference limit, and certainly a narrower CI.
Conclusions
A reference interval for the IMR has been established in a population of patients aged 40-60 years with structurally normal hearts, who are considered to be representative of the general population. The IMR was not related to sex, age or any of the other variables tested, suggesting that this reference range can be applied to the general population.
| Impact on daily practice A reference interval is mandatory for evaluating the use of IMR in further clinical studies. Even though the present study establishes a reference interval for IMR as being 7.3 - 27.2 in healthy individuals aged 40-60 years, it is still too early to predict its impact on day-to-day practice. |
Acknowledgements
The study was performed in and funded by the Department of Cardiology, Oslo University Hospital, Oslo, Norway. We thank the head of the department, the cardiac catheterisation and electrophysiological laboratory staff members, and the nurses in the Department of Cardiology at Oslo University Hospital Rikshospitalet (National Hospital of Norway) for their assistance in performing the study.
Conflict of interest statement
O.G. Solberg has received personal lecture fees from St. Jude Medical, Inc. E. Kongsgaard has served on an advisory board for St. Jude Medical, Inc. and has received research support from Medtronic, Inc. L. Aaberge has been a proctor for Edwards Lifesciences on TAVI. The other authors report no conflicts of interest relevant to the submitted work.

