Introduction
Leaflet thrombosis of bioprosthetic aortic valves is an increasingly recognised complication1. Subclinical leaflet thrombosis is more commonly observed following transcatheter aortic valve implantation (TAVI) and valve-in-valve (ViV) procedures than in surgical aortic valve replacement1. Anticoagulation therapy with warfarin, as compared with dual antiplatelet therapy, was associated with a decrease in the incidence of reduced leaflet motion in these patients1. However, bleeding is a complication of anticoagulation therapy, and recurrence of reduced leaflet motion has been observed following discontinuation of the anticoagulation therapy. Therefore, minimising the thrombogenicity of transcatheter aortic valves (TAVs) besides the pharmacological prevention methods is essential to ensure optimal long-term valve function. We have previously investigated the underlying flow-mediated mechanism that facilitates thrombus formation on TAV leaflets by computational modelling2. As another step towards minimising the thrombogenicity of TAVs, the present work aims to study the effects of transcatheter laceration of degenerated bioprosthetic valve leaflets, also known as the BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction) procedure3, on the likelihood of leaflet thrombosis following ViV procedures (Figure 1A). Although BASILICA was primarily developed to prevent coronary artery obstruction, the present work aims to study the role of BASILICA in diminishing the risk of leaflet thrombosis by increasing blood washout (i.e., decreasing blood stasis) on the surface of the TAV leaflets. We focused our study on ViV procedures with TAVs with intra-annular design that represents the worst-case scenario in terms of blood stasis on the TAV leaflets (Figure 1B) compared to supra-annular TAV design (Figure 1C).
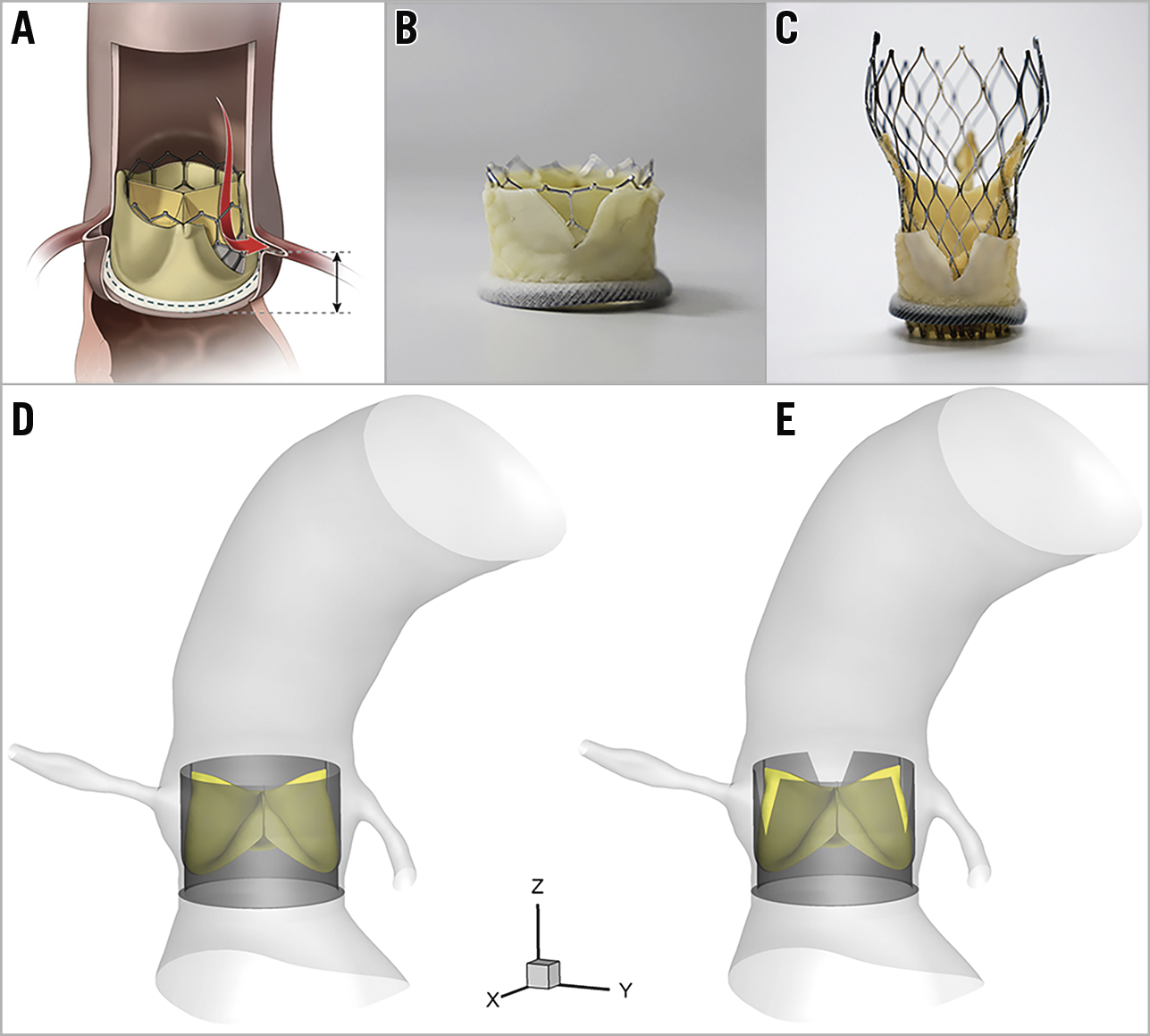
Figure 1. BASILICA technique and computational models. A) After BASILICA, blood flows through the open cells of the TAV. B) 23 mm SAPIEN 3. C) 26 mm Evolut™ PRO (Medtronic, Minneapolis, MN, USA) implanted in a 25 mm Mitroflow after the leaflet is cut with a scalpel to mimic BASILICA (Reprinted with permission from Khan et al3). In addition, computational models without (D) and with (E) bioprosthetic leaflet laceration.
Methods
Detailed flow investigation on TAV leaflets is a challenge in real-world clinical practice due to the limited temporal and spatial resolution of the currently available imaging modalities. Therefore, a fluid-solid interaction (FSI) modelling approach was used to simulate the three-dimensional (3D) unsteady flow field of a TAV in a patient-specific geometry. The computational modelling approach can also overcome the inherent limitations of in vitro experimental studies (e.g., lack of optical access to the neo-sinus region) for obtaining 3D flow patterns in the vicinity of TAVs. Patient-specific anatomic data were extracted from computed tomographic angiography images of a normal aortic root. Once the patient-specific geometry was obtained, two different computational models of a TAV based on a 26 mm Edwards SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) design implanted in a 29 mm Mitroflow surgical bioprosthesis (Sorin Group Inc., Saluggia, Italy) were developed without bioprosthetic leaflet laceration (Figure 1D), and with bioprosthetic leaflet laceration (Figure 1E). Details of (i) the FSI method, (ii) blood washout visualisation using imaginary massless particles, and (iii) quantification of blood residence time (BRT) have been described in previous studies2.
Results
Figure 2 demonstrates sample snapshots of the simulated flow fields at multiple time instants in a cardiac cycle in the ViV model with no bioprosthetic leaflet laceration (Figure 2A) and with bioprosthetic leaflet laceration (Figure 2B). The flow structure is shown by the contours of the velocity magnitude in the mid-plane cutting through the TAV. The configuration of the massless particles, that were initially released on the TAV leaflets for flow visualisation, is shown by black dots. A significant difference was observed between the two models in the flow field in the sinus region. In the systole, as shown in the first four columns of Figure 2B, a significant number of the particles in the BASILICA model went through the split leaflets and moved directly towards the coronary arteries. Moving image 1 clearly shows the movement of the massless particles.
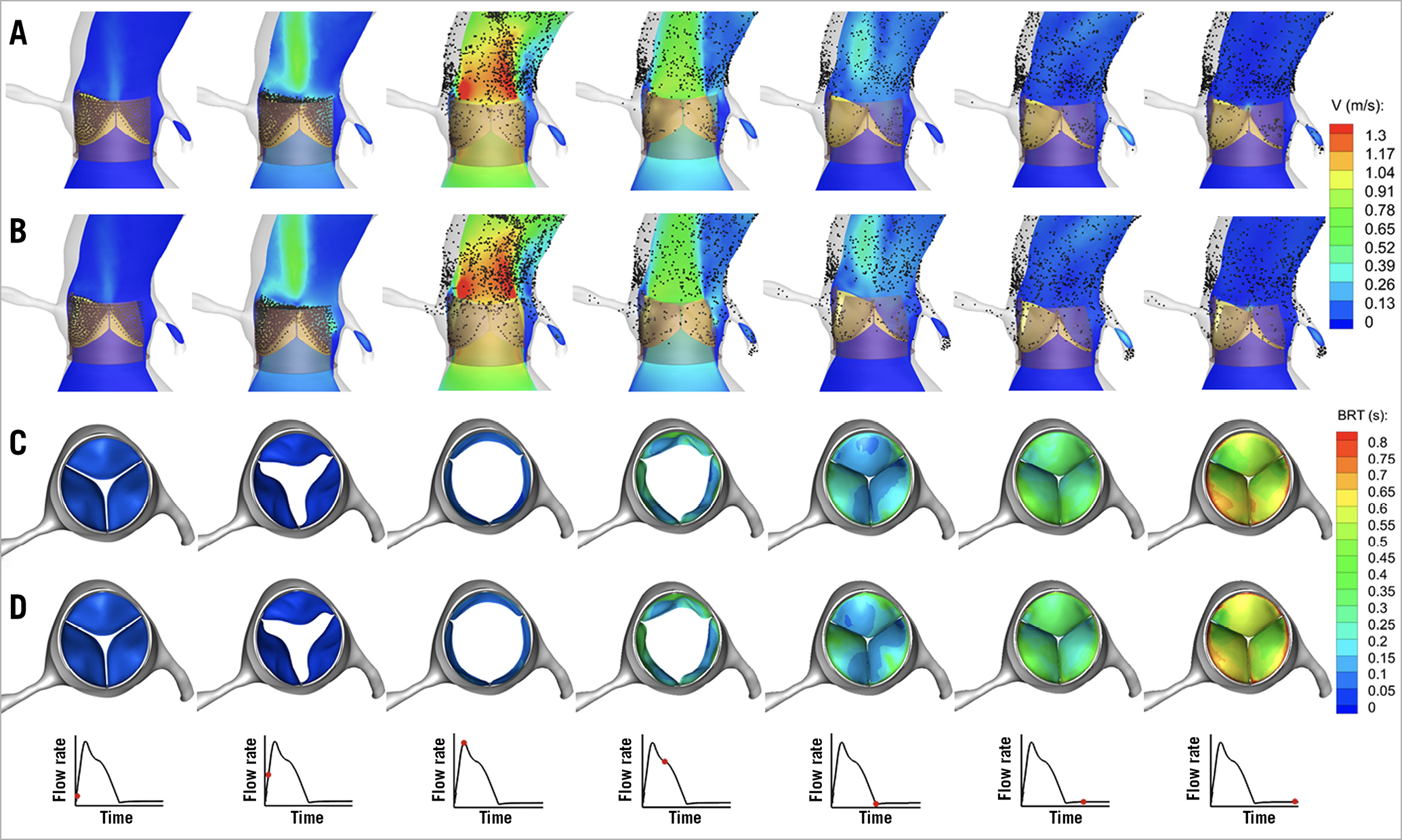
Figure 2. Snapshots showing the configuration of the flow field. A) ViV model with no bioprosthetic leaflet laceration. B) ViV model with leaflet laceration. In addition, snapshots showing the time evolution of the contours of BRT on the valve leaflets from top view during a complete cardiac cycle for ViV model with no bioprosthetic leaflet laceration (C) and ViV model after BASILICA (D).
In addition, we observed a difference in blood stasis on the TAV leaflets between the two ViV models. Figure 2C and Figure 2D show the contours of BRT from a top view on the three leaflets of the two ViV models. At the end of diastole, BRT was higher for the ViV model without bioprosthetic leaflet laceration than in the BASILICA model. The contours of BRT show that the areas that exhibit high BRT (shown in red) were concentrated near the fixed boundary of the leaflets. Furthermore, Moving image 2 clearly shows the distribution and magnitude of BRT in the two models. At the end of diastole, the average BRT on the surface of three leaflets in the BASILICA model was 9.8% less than that in the ViV model with no leaflet laceration. Acute thrombogenesis will occur on foreign surfaces of the TAV leaflets. The characteristic time of platelet activation is suggested to range from 0.1 s to 0.5 s4. In this study, areas of BRT more than 0.5 s that are highly prone to platelet activation were compared between the two models. On the left coronary leaflet, the areas with high BRT (TR >0.5 s) reduced considerably from 50.5% in the ViV model without leaflet laceration to 37.3% in the ViV model with leaflet laceration. In other words, BASILICA can effectively reduce the areas with high BRT on the aortic surface of left coronary leaflets that are prone to platelet activation by 13.2%. On the right coronary leaflet, the areas with high BRT (TR >0.5 s) reduced from 72.2% in the ViV model without leaflet laceration to 67.9% in the ViV model with leaflet laceration. No reduction in areas of high BRT was observed in the non-coronary leaflet.
Discussion
A number of patient-related factors such as inadequate antithrombotic therapy, poor left ventricular systolic function, or aortic root morphology can be considered responsible for leaflet thrombosis of TAVs. In addition, several device-related risk factors could potentially play a crucial role in leaflet thrombosis following ViV procedures. Personalised computational modelling of ViV procedures has the potential to provide a mechanistic explanation for TAV thrombosis on a case-by-case basis and fill the knowledge gap by computing detailed blood flow characteristics from which metrics such as BRT can be derived. The results of the present study indicate that BASILICA can reduce the areas with high BRT on the aortic surface of left and right coronary leaflets and potentially reduce the risk of leaflet thrombosis.
Limitations
This study was focused on TAVs with intra-annular design. However, the increased blood washout following BASILICA is expected to be more pronounced in supra-annular TAVs and in intra-annular TAVs without a Dacron sheet covering the base of the leaflets.
Conclusion
In summary, the results of the current study demonstrate that BASILICA can increase blood washout on the surface of TAV leaflets and reduce the areas with high BRT on the left coronary and right coronary leaflets following ViV implantation. Therefore, BASILICA has the potential to reduce the risk of leaflet thrombosis in selected ViV patients at high risk of leaflet thrombosis. Further prospective studies are motivated from the current single-case study to evaluate the role of BASILICA in reducing the risk of leaflet thrombosis following ViV and TAVI procedures.
|
Impact on daily practice The prevalence of ViV procedures in either failed surgical or transcatheter bioprosthetic heart valves is expected to increase dramatically with an ageing population. BASILICA has the potential to reduce the risk of leaflet thrombosis in selected ViV patients at high risk of leaflet thrombosis. |
Conflict of interest statement
D. Dvir reports consulting fees from Edwards Lifesciences, Medtronic, and St. Jude. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Movement of massless particles. A) Movement of the massless particles in the ViV model without bioprosthetic leaflet laceration during a cardiac cycle. B) Movement of the massless particles in the BASILICA model with bioprosthetic leaflet laceration during a cardiac cycle.
Moving image 2. Distribution and magnitude of blood residence time (BRT). A) Distribution and magnitude of BRT in the ViV model without bioprosthetic leaflet laceration during a cardiac cycle. B) Distribution and magnitude of BRT in the BASILICA model with bioprosthetic leaflet laceration during a cardiac cycle.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Movement of massless particles. A) Movement of the massless particles in the ViV model without bioprosthetic leaflet laceration during a cardiac cycle. B) Movement of the massless particles in the BASILICA model with bioprosthetic leaflet laceration during a cardiac cycle.
Moving image 2. Distribution and magnitude of blood residence time (BRT). A) Distribution and magnitude of BRT in the ViV model without bioprosthetic leaflet laceration during a cardiac cycle. B) Distribution and magnitude of BRT in the BASILICA model with bioprosthetic leaflet laceration during a cardiac cycle.

