Abstract
Aims: To assess the performance of the everolimus-eluting stent (EES) versus cobalt chromium bare-metal stent (BMS) in the setting of primary percutaneous coronary intervention for treatment of patients presenting with ST-segment elevation myocardial infarction (STEMI). The implantation of a drug-eluting stent in the setting of an acute myocardial infarction is still controversial. In several registries this clinical scenario has been associated with the development of stent thrombosis. The EES has demonstrated to reduce the stent thrombosis rate as compared to paclitaxel-eluting stent in randomised controlled trials, mainly performed in patients in stable clinical conditions. There are however few data regarding the effectiveness of EES in the context of STEMI.
Methods and results: This is an investigator-driven, prospective, multicentre, multinational, randomised, single blind, two-arm, controlled trial (ClinicalTrials.gov number: NCT00828087). This trial, with an all comer design, randomises approximately 1,500 patients 1:1 to EES or BMS. Overall, any patient presenting with STEMI up to 48 hours who requires emergent percutaneous coronary intervention can be included. The primary endpoint is the patient-oriented combined endpoint of all-cause death, any myocardial infarction and any revascularisation at 1-year according to the Academic Research Consortium. Clinical follow-up will be scheduled at 30 days, six months, one year and yearly up to five years. No angiographic follow-up is mandated per protocol.
Conclusions: This trial with broad inclusion and few exclusion criteria will shed light on the performance of the second generation EES in the complex scenario of STEMI.
Acronym
EXAMINATION stands for a clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION trial.
Background and rationale
The implantation of a drug-eluting stent (DES) in the setting of ST-segment elevation myocardial infarction (STEMI) has been controversial since the advent of those devices in the clinical arena. Most randomised controlled trials (RCT) and registries in the STEMI population demonstrated both efficacy and safety of first generation DES as compared to bare metal stents (BMS).1-6 In addition to this, recent meta-analyses showed no differences in stent thrombosis (ST) at 1-year follow-up by the use of DES as compared to BMS in STEMI.7,8 Nevertheless, acute myocardial infarction has been identified as one of the most potent clinical predictors of subsequent ST in many clinical registries.9-14 This risk may be further exacerbated in the STEMI context in case the patient discontinues the antiplatelet therapy within the first year after stent implantation.15
Both RCT and clinical registries acknowledge some caveats worthy of being noted. Normally, strict inclusion and exclusion criteria are applied in most RCTs leading to a highly selected population which is finally included. Therefore, generalisability of the results in the real world population may become, at the very least, debatable. Conversely, clinical registries barely exhibit inclusion and exclusion criteria, but may suffer from selection bias and under-reporting of events. Recently, RCTs with all-comers designs, allowing for wide inclusion and few exclusion criteria, are aimed at overcoming this lack of representation of real-world population in clinical studies.16,17 However, a recent single centre analysis from two all-comers RCTs showed that only 48% of the total number of patients screened was actually included in a trial of this type.18 Thus, an ideal scientific scenario would include an RCT with an all-comers design with the inclusion of the greatest majority of patients with STEMI with a complete follow-up during the entire study period.
The advent of second generation DES that release everolimus via a permanent fluorinated polymer, showed clinical improvements in both efficacy and safety as compared with first generation DES. This benefit was essentially observed when compared to paclitaxel-eluting stents.19,20 Currently, few data exists regarding the safety and efficacy of this type of second generation DES in the high-risk group of patients with STEMI.
We therefore developed this RCT with an “all-comers” design with the aim of evaluating the performance of everolimus-eluting stents (EES) in the complex setting of STEMI and to provide data that could be generalisable to a real-world population.
Methods
Study design
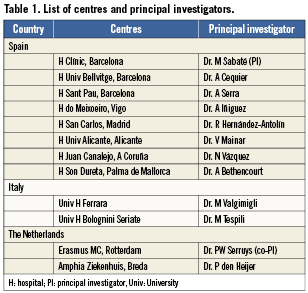
This is a multicentre, multinational, prospective, randomised, two-arm, single-blind, controlled trial performed in patients with STEMI (ClinicalTrials.gov number: NCT00828087). This study is an investigator-initiated trial and the promoter of the trial is the Spanish Society of Cardiology. The flow chart of the trial is described in Figure 1. Approximately, a total of 1,500 patients will be enrolled in the study within a period of 18 months, and will be randomised 1:1 to one of the two treatment arms: EES (Xience™ V stent; Abbott Vascular, Santa Clara, CA, USA) vs. a cobalt-chromium BMS (Multilink- Vision® stent; Abbott Vascular, Santa Clara, CA, USA). The allocation schedule is based on computer-generated random numbers. The randomisation is in blocks of four or six patients (randomly), stratified by centre and centralised by telephone. All centres submitted and received the approval of their Medical Ethics Committee for the protocol and for the informed consent. All patients must sign the informed consent before being included in the trial. The study is conducted in compliance with the protocol, the Declaration of Helsinki, BS EN ISO 14155 Part 1 and Part 2, and applicable local requirements. Twelve centres in three countries are involved in the trial. The list of centres is presented in Table 1.

Figure 1. Flow-chart of the trial.
Patient selection
This trial presents an all-comers design allowing broad inclusion and few exclusion criteria. Specifically, as inclusion criteria for these patients, after signing the informed consent, they can be included if suffering from STEMI up to 48 hours after the onset of symptoms, which requires emergent percutaneous coronary intervention (PCI), and with vessel sizes ranging between 2.25 mm and 4.0 mm to allow for the implantation of currently available stents. In order to allow for broad patient inclusion, in case the patient is unable to provide written informed consent (e.g., cardiogenic shock, cardiac arrest, etc.), written assent from a legally acceptable representative will be accepted to facilitate enrolment. Once, in the investigator’s opinion, the patient is capable of understanding the process and capable of signing the consent form, written consent will be signed from the patient.
In this way the patient can fall into one of the following categories according to current guidelines21: STEMI <12 hours after the onset of symptoms (namely, primary PCI); rescue PCI after failed thrombolysis; PCI indicated early (<24 h) after effective thrombolysis; and, patients presenting late (“latecomers”) with STEMI (>12 h<48 h after the onset of symptoms).
Patients in cardiogenic shock can also be enrolled in the trial. Exclusion criteria are age <18 years; pregnancy; patients with known intolerance to aspirin, clopidogrel, heparin, stainless steel, everolimus or contrast material; patients on chronic treatment with anti-vitamin K agents; STEMI secondary to stent thrombosis. The only anatomic exclusion criterion is the above-mentioned vessel size larger than 4.0 mm or smaller than 2.25 mm.
Stent types
The EES, the Xience™ V stent is a balloon expandable stent manufactured from a flexible cobalt chromium alloy with a multicellular design and 0.0032 inch strut thickness which is coated with a thin (7.8 μm) non-adhesive, durable, biocompatible acrylic polymer and fluorinated copolymer releasing everolimus.22 Everolimus (40-O-[2-hydroxyethyl]-rapamycin), a semisynthetic macrolide immunosuppressant, inhibits growth factor-stimulated cell proliferation by causing cell-cycle arrest in the late G1 stage, thereby suppressing neointimal formation.23 The comparator will be the cobalt chromium balloon expandable Multilink Vision® BMS also from Abbott Vascular. The design of both platforms (EES or BMS) is the same and corresponds to that of the Multilink Vision® stent.
Study endpoints
The primary endpoint of the study is the patient-oriented combined endpoint of all-cause death, any myocardial infarction and any revascularisation at 1-year according to the Academic Research Consortium (ARC).24
The secondary endpoint of the study includes the stent-oriented combined endpoint of cardiac death, target vessel myocardial infarction and ischaemia-driven target vessel revascularisation at one year.24 In addition, the following secondary endpoints are also examined at 1-year and yearly up to five years: all cause and cardiac mortality; recurrent myocardial infarction; target lesion revascularisation; target vessel revascularisation; stent thrombosis (according to the ARC definitions24); device and procedure success; major and minor bleeding.
Definitions
All-cause death includes cardiac death, vascular death and non-cardiovascular death. Cardiac death was defined according to ARC definition24 as any death due to proximate cardiac cause (e.g., MI, low-output failure, fatal arrhythmia), unwitnessed death and death of unknown cause, and all procedure-related deaths, including those related to concomitant treatment.
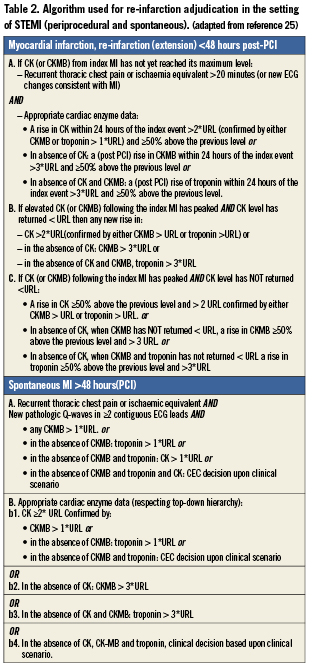
Recurrent myocardial infarction is defined according to the WHO extended definition.25 Both periprocedural and spontaneous myocardial infarction were assessed. For both situations a dedicated algorithm was used in the adjudication process (Table 2).
All types of revascularisation, including target lesion, target vessel and non-target vessel revascularisations, are taken into account. Addionally, revascularisation is considered ischaemia-driven if associated with any of the following: non-invasive positive functional ischaemia study (e.g., exercise testing or equivalent tests) or invasive positive functional ischaemia study (e.g., fractional flow reserve or coronary flow reserve); ischaemic symptoms and an angiographic minimal lumen diameter stenosis ≥50% by on-line quantitative coronary angiography (QCA); or, diameter stenosis ≥70% by on-line QCA without either ischaemic symptoms or a positive functional study.
Stent thrombosis is classified as acute, subacute, late and very late; and, as definite, probable and possible according to ARC.24
Clinical device success is defined as successful delivery and deployment of the first inserted study stent (in overlapping stent setting a successful delivery and deployment of the first and second study stent) at the intended target lesion and successful withdrawal of the stent delivery system with attainment of final residual stenosis of less than 50% of the target lesion by QCA (by visual estimation if QCA unavailable), without use of a device outside the assigned treatment strategy. Clinical procedure success is defined as clinical device success without the occurrence of ischaemia-driven major adverse cardiac event during the hospital stay with a maximum of first seven days post index procedure. In dual lesion setting both lesions must meet clinical procedure success.
Bleeding complications are categorised as major or minor according to TIMI definition.26 Besides, it is considered post hoc adjudication according to BARC criteria.27
Index and staged procedures
At the index procedure, patients receive appropriate anticoagulation and other therapy according to standard hospital practice. Either unfractionated heparin or bivalirudin may be used for procedural anticoagulation. The use of glycoprotein IIb/IIIa inhibitors is left to the discretion of the investigator. Aspirin (loading dose 250-500 mg) and clopidogrel (loading dose of at least 300 mg) should be administered before PCI for those patients not on chronic antiplatelet treatment. Ticlopidine at a dose according to standard hospital practice can be given if the patient was allergic to clopidogrel. Neither prasugrel nor ticagrelor were approved during the recruitment period. Patients must continue with clopidogrel for at least one year (75 mg per day) and with aspirin (100 mg) indefinitely. PCI is performed according to standard techniques in thrombotic scenarios. Manual thrombectomy followed by direct stenting is the recommended technique in this setting, although other devices could also be used if considered necessary. Full lesion coverage has to be ensured by implantation of one or multiple stents. Operators are instructed to insert only the assigned stent type at the index procedure. No mixture of stent types is permitted in a given target lesion unless the assigned study stent cannot be placed in which case the operator should crossover to another stent or device. There is no limitation to the number of vessels and lesions to be treated.
Patients with multivessel disease needing staged PCI procedure may also be included. This had to be recorded in the case report form. A recommendation is made to implant the same stent type, as per randomisation, in all staged lesions. However, in case of specific clinical or anatomical conditions (e.g., chronic total occlusion, proximal left anterior descending, long lesion, diabetic patient) operators can eventually consider using the type of stent that they consider best for patient’s condition, regardless of the stent type implanted at the index procedure. Importantly, all staged procedures need to be completed within the first month following discharge. Otherwise, this revascularisation will be considered as an event.
Blood sample analyses including biomarkers of myocardial damage (creatine kinase, creatine kinase MB or troponin) are obtained before the index procedure, within the 12 hours and within 24 hours after the procedure. ECG is taken before the procedure, within 30-60 min after the procedure and every 24 hours until discharge. Additional blood samples and ECG can be obtained if clinically considered or in the presence of recurrent symptoms. At the time of the staged procedure pre- and post-procedural blood samples and ECG are also obtained to rule out periprocedural complications.
Follow-up
Patients will be followed up to five years after the index procedure. The follow-up will include a clinical visit or telephone contact regarding cardiovascular drug use, hospitalisations, invasive or noninvasive diagnostic tests and clinical events at 30 days, six months and one year, and yearly up to five years. No angiographic follow-up is mandated per protocol. Thus, any follow-up angiography will be clinically indicated.
Study committees
Serious adverse events (events leading to serious disability or admission to hospital, life-threatening events or death) are periodically reviewed and analysed by an independent Data and Safety Monitoring Board (DSMB). Members of this board are blinded, i.e., unaware of the patients’ treatment allocation, not affiliated with any of the involved centres, nor are they participating in the trial, and will declare any conflicts of interest should they arise. The DSMB are responsible for making recommendations to the Steering Committee regarding endpoint analyses and any potentially significant patient safety-related observations.
The Clinical Event Committee (CEC, Cardialysis, Rotterdam, The Netherlands) consists of cardiologists not participating in the trial. All adverse events are reviewed, adjudicated and classified by the CEC. The CEC members are also blinded to the patients’ treatment allocation and trial results.
The Steering Committee is responsible for overseeing the scientific and operational aspect of the study. This committee regularly meet to monitor patient inclusion, non-compliance with the investigational plan at individual centres, to review and act upon recommendations of the DSMB, and to determine policy regarding any publications arising from data generated from the performance of the study.
Statistical analysis
All analyses are performed on the intention-to-treat population. The intent-to-treat population consist of all patients randomised to the study, regardless of the treatment actually received. Additionally, the analysis are performed by the treatment finally received (if different from allocated by randomisation).
The overall sample size for the study of 1,500 patients is based on the primary endpoint of all-cause death, any myocardial infarction and any revascularisation at 1-year. The sample size calculation is based on a 2-sided type I error rate α=0.05, randomisation ratio 1 (EES arm): 1 (BMS arm) and a statistical power of at least 86% to detect approximately 30% reduction in the rate of the primary endpoint at 1-year from 20.5% in the control group to 14.5% in the XIENCE V group. To estimate the rate of events in the BMS arm, we used the data available from all-comers Registries (Research, T-Search) and meta-analysis of randomised controlled trials that included patients with STEMI.28,29.
Statistical testing of the primary endpoint will be performed with the log-rank test at a two-sided 0.05 significance level for the comparison of EES arm to BMS arm. Adjustment for important covariates, such as centre, diabetes status will be used to refine the unadjusted analysis. Analyses of other study endpoints will be descriptive in nature, using point estimates and their respective two-sided 95% confidence intervals. Count variables will be presented as percentages, continuous variables as means (medians and interquartile ranges whenever appropriate). For time-to-event variables, survival curves will be constructed using Kaplan-Meier estimates, and log rank test results will be displayed for descriptive purposes only.
Subgroup analyses are pre-specified for the following variables: gender; age>70 vs. ≤70; diabetes; primary PCI vs. no primary PCI; TIMI post PCI<3 vs. 3; concomitant treatment with glycoprotein IIb/IIIa inhibitors; use of aspiration thrombectomy catheters; multivessel disease vs. single vessel disease; ischaemia time <3 hours vs. ≥3 hours; time first medical contact-first device in <120’; ejection fraction <30% vs. ≥30%; left anterior descending as infarct-related artery. These variables have been demonstrated to be classical predictors of outcomes after STEMI or of potential benefit in this context (e.g., use of thrombectomy catheter).
During the recruitment period, all PCI in the setting of STEMI performed at the institutions involved will be collected, as well as the reasons why these procedures were not included in the trial.
Discussion
This multicentre trial is aimed to assess the efficacy and safety of EES in the setting of STEMI in a global context, representative of the real world.
The outcomes after STEMI may be the result of multifactorial causes, not always related to the stent implanted at the index procedure. However, it is often difficult to discern the potential relationship between the baseline stent implanted and the actual event during follow-up. Sudden death after STEMI, for example, may be related to different causes such as, stent thrombosis; recurrent myocardial infarction located either at target or at non-target vessel; fatal arrhythmia related to a low residual ejection fraction; mechanical complication such as cardiac rupture; stroke related to medication needed after stent implantation among others. For all these reasons, in the scenario of STEMI, a broad concept of the patient-oriented endpoint makes probably full sense. This composite primary endpoint that included all-cause death, all myocardial (re)-infarction and all repeat revascularisation procedures24 may therefore reflect the complex interplay between device performance, revascularisation strategy, procedural aspects, secondary prevention, residual left ventricle function, and other patient conditions. In the event of multivessel disease that needs to be treated at a later stage, a recommendation to use the same stent as per randomisation is made. However, crossing over the stent is allowed, if, in the investigator’s opinion, this is the best option from the clinical point of view (e.g., DES rather than BMS for proximal left anterior descending artery, diabetic, long lesions, among others). The downside of this analysis includes the potential dilution effect of the crossover patients in the any revascularisation component of the primary endpoint. In this regard, it is expected that at staged procedure, all high-risk lesions may eventually be treated with EES rather than with BMS. From the patient selection point-of-view, this strategy avoids some selection bias that could be produced in the event of a restriction on the use of a stent at the staged procedure. Further, the strategy of recommending, but not forcing the stent to be implanted at the staged PCI, truly reflects what naturally occurs in the real world. This clearly assures the generalisability of the future results of the study. Finally, to certainly identify the potential added value of the EES over BMS, a dedicated stent-oriented endpoint is defined following ARC recommendations24 as secondary endpoint. This includes the composite of cardiac death, target vessel myocardial infarction and ischaemia-driven target lesion revascularisation at 1-year follow-up. From the scientific point-of-view, the design of an all comers trial allows the assessment of both efficacy and effectiveness of a new technique. The former is classically assessed by randomised controlled trials with restrictive inclusion/exclusion criteria, whereas the latter by clinical registries of real world patients. Under the concept of all comers, we may tackle both sides of the spectrum by controlling all confounding variables by randomisation and letting the results be representative of the vast majority of patients (“real world”). In order to be able to accomplish this, finally the majority of patients presenting with STEMI and arriving at the catheterisation laboratory during the study period should be recruited for the trial. Therefore, patients with STEMI arriving <12 h after the onset of symptoms (“primary PCI”), rescue PCI and PCI after successful thrombolysis can be included. Additionally, patients arriving late (>12 h <48 h) but with clinical indication of emergent PCI (persistent chest pain, persistent ST elevation, etc.) can also be included. A recent meta-analysis30 demonstrated potential benefit in mortality of PCI in latecomers after STEMI. As a result, the EXAMINATION trial should represent the paradigm of an “all-comers most included” randomised trial.
STEMI still represents an off-label indication for the use of DES, despite results of RCTs of first generation DES. The concerns raised by clinical registries have probably induced the FDA to keep considering this clinical condition as off-label31. Both the anatomical substrate and the pro-thrombotic milieu occurring in STEMI may delay endothelialisation; in pathological studies it was observed, for example, a delayed healing in first generation DES implanted in culprit lesions from STEMI patients as compared to those implanted in lesions from stable patients.32 Additionally, late acquired incomplete stent apposition has more often been observed after first generation DES than after BMS implantation both in stable conditions33 and in the context of STEMI.34 These factors, together with hypersensitivity reaction to the polymer of first generation DES, have been suggested as causes of stent thrombosis.35,36 Conversely, second generation EES may confer an improved safety and efficacy profile. Several RCTs have compared the efficacy and safety of EES versus first generation DES, demonstrating for EES a rate of stent thrombosis comparable to sirolimus-eluting stent and significantly lower than that of paclitaxel-eluting stent.19,20 Most patients included in these trials presented stable clinical status. Thus, results for these cannot be extrapolated to the pro-thrombotic scenario of the STEMI.
The eventual benefit of the EES (as compared to either paclitaxel-eluting stent or BMS) may be related to the presence of relatively thin struts (96×96 µm2) and the fluorinated copolymer used to elute the everolimus. This copolymer is composed of vinylidene fluoride and hexafluoropropylene monomers that may confer a certain degree of thromboresistance and haemocompatibility35. This benefit can even be extended to incompletely apposed or overlapping stents, where the presence of the copolymer resulted to be less thrombogenic than the BMS with complete absence of polymer as previously demonstrated in a bench study.37
Results of this trial may entail clinical implications. Corroboration of the results already obtained in the non-STEMI population, may dissipate the concerns about the use of EES in a high-risk clinical context, such as STEMI. Nevertheless, to change the paradigm, the investigators have to be able to include the vast majority of patients arriving at the hospital during the study period, with the fewest number of missing patients during follow-up. In such a scenario, results will be generalisable to the real world population. In the same way, concomitant medications, procedural aspects and logistics (i.e., time from first medical contact to first device in) should be in line with current guidelines to demonstrate the added value of the stent to the standard treatment. Finally, the absence of mandated angiographic follow-up will mimic clinical practice.
Conclusion
This multicentre randomised investigator-initiated trial evaluates the performance of the EES as compared to the conventional cobalt chromium BMS in the setting of STEMI. The all comer design, the patient-oriented nature of the study with a clinically-based primary endpoint24 and the absence of mandated angiographic follow-up will allow the results to be seen as highly representative of the real world population with STEMI.
Disclosures
This trial was partially funded by an unrestricted grant from Abbott Vascular to the Spanish Heart Foundation.
Conflict of interest statement
B. Backx is an employee of Cardialysis BV. The other authors have no conflict of interest to declare.

