X-ray technology is essential for all invasive cardiovascular procedures. This energy has ionising radiation properties that may cause harm1-3. In recent decades we have witnessed great improvement in all aspects of the procedure; but the use of ionising radiation has remained unchanged and there have been only insignificant improvements in this important aspect of the procedure.
Advances in devices and techniques have led to increasingly more complex percutaneous coronary interventions (PCI) and catheter ablations, new structural heart disease interventions, new approaches to peripheral vascular disease, and numerous other procedures performed under fluoroscopy. The procedure is now safer, more predictable and is more accessible in different parts of the world. As a result, more interventions are being done worldwide, and these are increasingly complex with more radiation.
Occupational radiation exposure, and the orthopaedic complications from wearing the heavy leaded aprons necessary to limit such exposure risk, have become a major concern among physicians performing interventional procedures. The operator works standing and wearing lead, and this is done repeatedly, almost on a daily basis, over many years. As a result, especially after several years of work, operators will frequently develop orthopaedic problems. Interventional cardiologists are significantly more likely to have cervical disc disease and multiple spinal levels of disc involvement, and are nearly twice as likely to miss work due to orthopaedic complaints compared to other physician groups4. Fortunately, the orthopaedic problems can usually be relieved by rest and other conservative methods. The more serious potential danger in interventional cardiology is radiation5. Several reports have found that the dosages of ionising radiation experienced by interventional cardiologists are the highest ones registered amongst medical staff using x-rays6.
The biological effects of radiation pose a potential threat to medical personnel. These can be dependent on the total dose (deterministic effects), or independent of dose (stochastic effects). It is evident that smaller doses of radiation, which are generally encountered in many diagnostic procedures, may not exceed the threshold dose for deterministic effects. However, a probability still exists for stochastic effects.
In parallel, in recent years we have witnessed an unprecedented shift from the traditional femoral artery approach to the transradial (TR) artery approach. The main reasons for this are the reduction in vascular complications with TR access, improvements in ambulation time, length of post-procedure hospital stay and simplified same-day PCI discharge.
In this issue Park et al7 review the literature regarding radiation exposure associated with the TR approach. Although several studies have reported an increase in radiation exposure to both operator and patient with TR compared with femoral access, others have reported findings suggesting no significant difference, even reporting decreased exposure with TR access. Overall, increased exposure appears likely with TR access; however, in consideration of the many benefits reported with TR access, exposure remains only one of many considerations when deciding between routes of access.
We are facing more clinical indications for invasive procedures and new areas of interventions with the use of fluoroscopy. We already treat more complex coronary disease. It is estimated that a greater proportion of cases will be done using the TR approach associated with more radiation exposure. Therefore, it is expected that exposure to radiation will increase despite the industry’s challenge to reduce the amount of radiation. As a result, interventional operators today and in the future face greater occupational risks than ever before.
The central dogma of radioprotection is that the biological effects of ionising radiation are a direct consequence of DNA damage occurring in directly irradiated cells. Therefore, for many years, based on this fundamental law of radiobiology, the brain was considered a paradigm of a highly differentiated organ with low mitotic activity, and thus considered radio-resistant. The same concept was considered true regarding the lens of the eye. These assumptions have been challenged by recent evidence4,5. Interventional cardiologists working in a high-volume catheterisation laboratory have higher levels of somatic DNA damage when compared with clinical cardiologists working outside the catheterisation laboratory8-10.
A strong dose-response relationship was found between occupational exposure to radiation and the prevalence of radiation-associated posterior lens changes. Relative risks of lens opacity were 5.7 (95% CI: 1.5-22) for interventional cardiologists, and 5.0 (95% CI: 1.2-21) for nurses working in catheterisation laboratories11.
While the body and the thyroid are protected with lead, and special glasses can protect the eyes, the head is completely exposed! Is there a real risk for head tumours in this growing field of medicine?
Ionising radiation is an established environmental cause of brain cancer5. Although direct evidence is lacking in contemporary fluoroscopy due to obvious sample size limitation, limited follow-up time and lack of focused research, anecdotal reports of clusters have appeared in the literature raising the suspicion that, for interventional cardiologists, brain cancer may be a professional disease12-21 (Table 1).
In Europe and North America the incidence for glioblastoma multiforme (GBM) is two to three cases per 100,000, and for symptomatic meningioma it is approximately two cases per 100,000 individuals. There are limitations of interpreting observations from a “cluster” of cases where the true “numerator and denominator” of cases falls within a total population and this must be emphasised. Solid evidence-based medicine in this field is lacking, and the cases could all be a simple matter of chance without any relationship to occupational exposure. However, it is unquestionable that a study with and without protection will never be done.
Of note is that interventional cardiologists are subjected to head dose radiation exposure 10 to 20-fold higher than the dose recorded beneath the apron. The annual exposure to a cardiologist’s head is in the range of 20-30 mSv22, or much higher if a ceiling-suspended screen is not used23. The left side of the operator is more exposed to radiation than the right side due to the usual layout of an interventional room, where the operator stands at the right side of the patient. Scattered radiation comes from the patient and is more intense when the x-ray tube is on his/her left. This implies that the lifetime estimated organ dose for a busy interventional cardiologist after 25 years of work in the catheterisation laboratory is in the order of magnitude of 1 to 3 Sv as scatter dose measured at the head with a dosimeter, with the brain dose (inside the skull) being approximately 20-25% of the external dose5.
We recently reported nine cases of brain cancer in interventional cardiologists20. Following this publication, we received voluntary reports, usually by email, of additional cases from around the world. An expanded series of 31 cases was published21. Since the last publication another three cases have been reported. All reported cases were from Europe and North America (Figure 1). The most recent data, as of October 2013, includes 34 physicians with brain and neck malignancies: 26 interventional cardiologists, two electrophysiologists and six interventional radiologists. All worked for prolonged periods (latency period 12-32 years, mean 23.5±5.9 years) in active interventional practice with exposure to ionising radiation in the catheterisation laboratory. Tumours included 17 (50%) cases of GBM, two (6%) astrocytomas, four (12%) other gliomas and five (15%) meningiomas. In 29/34 cases, data were available regarding the side of the brain involved: the malignancy was left-sided in 25 (86%) cases, midline in one and right-sided in three operators.
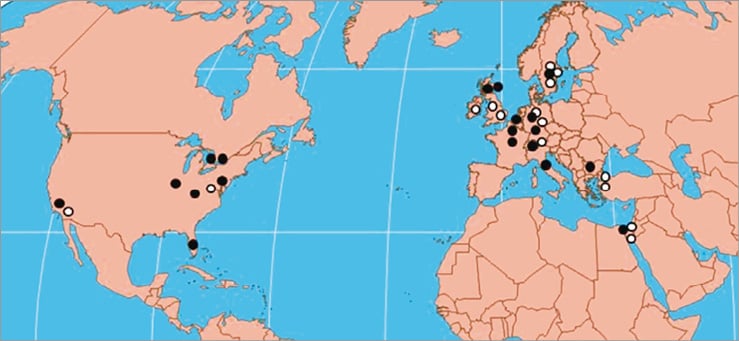
Figure 1. Geographical distribution of brain and neck tumour voluntary reports (adapted from Ref 21). Black circles are gliomas.
In the general population all these malignancies have equal distribution between the left and right side. If we perform a Fisher’s exact test comparing the 25 left and four non-left to a normal expected distribution in the general population (50% of each side), then, for a population of 29 subjects, the p-value is <0.01. Of legal importance, the brain tumour of one of those physicians was recently recognised by the legal authorities in his country as a professional disease related to his work and exposure to radiation in the catheterisation laboratory.
How can we decrease the exposure to radiation? In this issue we have a very interesting report in which a 0.5 mm lead equivalence cap covering the head reduced the dose to the head to ignorable levels, regardless of the use of a shield24. Karadag et al should be congratulated for this important work. They investigated the efficacy of a lead cap in radiation protection of the head using several protection options and found that this method blocked all radiation to the brain. However, there are still several points we need to address before we all adopt this type of head cover. For instance, how will a patient feel when his doctor enters the catheterisation laboratory dressed as an astronaut ready for a “space-walk”? Also, what are the long-term orthopaedic consequences of wearing this head cover, and will it increase spinal problems for the wearer (although its weight is minimal)?
A different approach, also reported in this issue, is the application of a simple lead apron covering the abdomen from the umbilicus and down, which significantly reduces radiation scatter. Radiation from the fluoroscopy tube is scattered by the patient while the cardiac intervention is underway and can reach the physician’s head. Interventional cardiology procedures performed via the TR approach are associated with longer fluoroscopy times and greater cumulative scatter radiation to the operator and staff.
Osherov et al25 folded a regular lead apron to create a rectangular shielded area with dimensions of 60×100 cm, and an attenuation equivalent to 0.5 mm of lead. In a phantom model using three common projections, this method was able to significantly reduce the operator’s exposure to scattered radiation (93% at 50 cm; 86% at 100 cm).
We as a community should make every effort necessary to decrease the potential hazards of radiation. Every effort must be made to keep the dose as low as reasonably achievable (ALARA) while at the same time keeping the benefit from diagnosis and optimal management as high as reasonably achievable (AHARA). Implementing best clinical practice using ionising radiation and monitoring and recording radiation exposure are essential steps that must be implemented by those providing the tests as well as by referring physicians.
Today, in most laboratories, overhead radiation shields, special glasses, thyroid shields, and leaded aprons are employed to reduce radiation doses to the operator. Less scatter from the patient will also decrease the exposure of the operator. Simple methods for reducing or minimising occupational radiation dose include minimising fluoroscopy time and the number of acquired images; using available patient dose reduction technologies; collimating; avoiding high-scatter projections; and wearing personal dosimeters. Effective use of these methods requires both appropriate education and training in radiation protection for all interventional cardiology personnel, and the availability of appropriate protective tools and equipment.
What else can be done apart from the head cap and lead apron covering the abdomen from the umbilicus and down reported here?24,25.
Less conventional solutions such as rolling or ceiling-mounted “weightless” aprons may also reduce pressures applied to the spine, but are not convenient for free movement and are not common in clinical practice4. Recently, a disposable bismuth-barium radiation shield drape (RADPAD®; Worldwide Innovations & Technologies Inc., Kansas City, KS, USA) was shown to reduce the dose of operator radiation by 23%26.
Will it be possible to work without lead aprons? The Trinity Radiation Protection System (ECO Cath-Lab Systems, Inc., Woods Cross, UT, USA) consists of a combination of fixed shields, radiation drapes, and interconnecting flexible radiation resistant materials creating a complete radiation protection environment for the operators, yet maintaining full and unimpeded contact with the patient and total control of all operational elements of the catheterisation equipment. A recent pilot trial showed that radiation was significantly lower and may, in the future, obviate the need for leaded aprons27.
Perhaps the future will see us working in remote stations using robotically-enhanced coronary intervention systems. Recently, one of these systems was demonstrated to be safe and feasible in performing PCI in 164 patients enrolled at nine different sites28.
Epidemiologic evidence for radiation-induced brain cancer in fluoroscopists is suggestive, but by no means conclusive. A review of cohort mortality studies among workers exposed to ionising radiation in U.S. nuclear programmes was reported in 1991 and reappraised in 2001, with 3.8 person-years of observation among 140,000 white male workers. The increased risk of brain malignancies was highly consistent, persistent, and stable, in the order of magnitude of 15-30%. As a consequence of these data, policy makers have identified brain cancer as a “specified” cancer potentially related to occupational exposures under the Energy Employees Occupational Illness Compensation Program Act30.
We cannot perform a procedure without x-rays and ionising radiation. However, we should remember that we are dealing with a double-edged sword that can potentially hurt the operator. The strikingly disproportionate reports of tumours of the left side of the brain, that region of the head known to be more exposed to radiation and least protected by traditional shielding, suggest, but do not establish, the possibility of a causal connection between occupational radiation exposure and brain cancer.
Conflict of interest statement
The author has no conflicts of interest to declare.

