
Abstract
Aims: We sought to compare clinical outcomes and procedural characteristics with transradial access (TRA) versus transfemoral access (TFA) in patients who were treated with PCI for left main (LM) coronary artery disease.
Methods and results: The EXCEL trial was a prospective, international, open-label, multicentre trial that randomised 1,905 patients with LM disease and SYNTAX scores ≤32 to PCI with everolimus-eluting stents versus coronary artery bypass grafting. The present analysis cohort consisted of 931 patients undergoing PCI with TRA or TFA, but not both. The primary endpoint was a composite of death, myocardial infarction (MI), or stroke at three years. Multivariable Cox proportional hazards regression was used to adjust for differences in baseline covariates. PCI in EXCEL was performed exclusively with TRA in 248 (26.6%) patients and with TFA in 683 (73.4%) patients. TRA patients were younger and less likely to have hypertension and chronic kidney disease. The mean number of vessels and lesions treated was higher in TFA patients, although the SYNTAX score was similar in both groups. Patients undergoing TRA and TFA had similar 30-day rates of TIMI major or minor bleeding (2.4% versus 3.8%, respectively, p=0.30). At three years, TRA and TFA patients had similar rates of the primary endpoint (15.7% versus 14.8%, adjusted HR 1.11, 95% CI: 0.73-1.69, p=0.64), as well as the individual rates of death, MI, stroke, ischaemia-driven revascularisation and stent thrombosis.
Conclusions: In the EXCEL trial, PCI of LM disease with TRA was associated with comparable early and late clinical outcomes to TFA.
Abbreviations
AKI: acute kidney injury
BARC: Bleeding Academic Research Consortium
CABG: coronary artery bypass grafting
EES: everolimus-eluting stent
HR: hazard ratio
LM: left main
MI: myocardial infarction
PCI: percutaneous coronary intervention
TFA: transfemoral access
TIMI: Thrombolysis In Myocardial Infarction
TRA: transradial access
Introduction
The common practice of attaining arterial access via the femoral artery (transfemoral access [TFA]) for percutaneous coronary intervention (PCI) has been challenged in recent years by the demonstration in randomised trials that transradial access (TRA) reduces vascular complications, bleeding, and perhaps even mortality1-6. However, much of the benefit was in patients with acute coronary syndromes, and some have argued that methodologic issues in these studies (including differences in antithrombotic regimens and operator experience) limit the validity of the results7. Nonetheless, relatively little has been reported on the outcomes of TRA in patients with complex or high-risk coronary artery disease. Modest-sized retrospective registries have compared outcomes of patients who underwent unprotected left main (LM) PCI using TRA versus TFA, in general reporting similar procedural success, shorter hospitalisation, reduced bleeding rates, and comparable long-term clinical safety and efficacy with TRA8-10. However, few data are available from prospective trials comparing these approaches in high-risk patients with LM disease.
We therefore sought to compare the early and late outcomes with TRA versus TFA in patients undergoing LM PCI from the large-scale, contemporary EXCEL (The Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trial11.
Methods
STUDY DESIGN AND STUDY POPULATION
The study design and primary results of the EXCEL trial have been described in detail previously11,12. In brief, EXCEL was a prospective, international, open-label, multicentre, randomised trial that compared PCI with the cobalt-chromium everolimus-eluting XIENCE stent (Abbott Vascular, Santa Clara, CA, USA) to coronary artery bypass grafting (CABG) in 1,905 patients with unprotected LM disease and low or intermediate SYNTAX scores (≤32)12. Key inclusion criteria were visually estimated LM stenosis ≥70% or ≥50% to <70% with non-invasive or invasive evidence of haemodynamic significance, and consensus among the members of the Heart Team regarding eligibility and equipoise for revascularisation with either PCI or CABG. The trial was approved by the institutional review board at each site, and all patients provided written informed consent.
The primary endpoint was the composite rate of death from any cause, stroke, or myocardial infarction (MI) at three years. The secondary endpoints were the same composite measure at 30 days, and the composite rate of death, stroke, MI or ischaemia-driven revascularisation at three years. The definitions of the component endpoints have been reported previously11. An independent central events committee reviewed and adjudicated all major primary and secondary endpoint events, as well as stent thrombosis and graft occlusion. Thrombolysis In Myocardial Infarction (TIMI)-scale bleeding13, Bleeding Academic Research Consortium (BARC)-scale bleeding14, and vascular complications (defined as peripheral distal embolisation [non-cerebral], arterial rupture, dissection, occlusion, stenosis, perforation, aneurysm or pseudoaneurysm, arteriovenous fistulas, large haematomas requiring vascular surgery or blood transfusion, or failure of percutaneous access-site closure resulting in interventional [e.g., stent-graft] or surgical repair) were site reported and monitored, but not centrally adjudicated. All patients have presently reached the three-year follow-up time point.
STATISTICAL ANALYSIS
For the present study, we analysed the outcomes of patients who were randomised to and subsequently underwent PCI with TRA versus TFA. Patients who had both TRA and TFA were excluded. Unadjusted comparisons of baseline and procedure characteristics, medical history, and clinical events were conducted using the χ2 test or Fisher’s exact test for binary variables, t-test or Wilcoxon rank-sum test for continuous variables, and log-rank test for time-to-event variables. To account for the differences in baseline characteristics according to vascular access, clinical outcomes were compared in Cox proportional hazards regression models adjusted by a propensity score for TRA vs. TFA use and stratified by US vs. non-US site. The propensity score was calculated by logistic regression using the following covariates: age, sex, chronic kidney disease, peripheral vascular disease, chronic obstructive lung disease, left ventricular ejection fraction, diabetes mellitus, body mass index, prior transient ischaemic attack or stroke, congestive heart failure, SYNTAX score (angiographic core laboratory assessed), hypertension, hyperlipidaemia, anaemia, and anticoagulation used during PCI (heparin or bivalirudin). The variables distal LM bifurcation involvement (versus isolated ostial/shaft stenosis) and planned two-stent LM PCI strategy were added, and the variable anticoagulation used during PCI was excluded from the linear models for procedure duration, contrast amount, and radiation dose. All p-values are two-tailed, and a p-value <0.05 was considered significant for all analyses. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
STUDY POPULATION
A total of 1,905 patients with LM disease were enrolled from 131 international centres, among whom 948 were randomised to PCI. PCI was the first procedure performed in 935 patients. Two PCI patients who were treated by both TRA and TFA, and two other patients in whom other access routes were used were excluded. Thus, the analysis cohort consisted of 931 PCI patients, 248 (26.6%) of whom were treated exclusively by TRA and 683 (73.4%) of whom were treated exclusively by TFA. The use of TRA varied considerably between countries. TRA for LM PCI was used in only 7.4% (20/272) of PCI patients enrolled in the USA compared with 33.7% (178/528) enrolled in Europe and 48.5% (49/101) in Canada (Supplementary Figure 1).
PATIENT CHARACTERISTICS AND PROCEDURES
As shown in Table 1, TRA patients were younger, more often presented with stable angina, and had less chronic kidney disease than TFA patients. As shown in Supplementary Table 1, the mean number of vessels and lesions treated was higher in TFA patients, although the SYNTAX score was similar in both groups. The guide catheter most commonly used was 6 Fr in the TRA group and 7 Fr in the TFA group. Bivalirudin was more commonly used in TFA procedures. No significant differences were present in the rates of procedural complications or duration of hospitalisation between the groups.
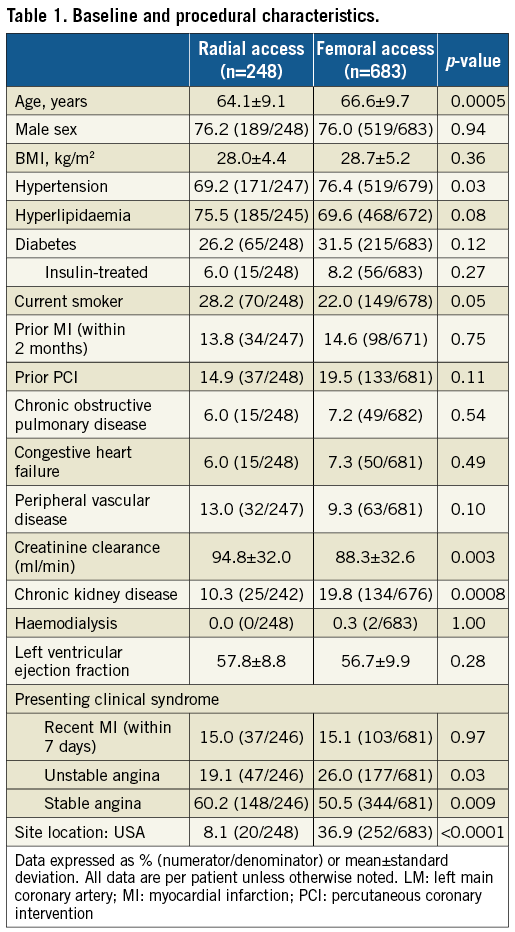
CONTRAST VOLUME, PROCEDURE DURATION, AND RADIATION DOSE
TRA was associated with less contrast use than TFA (233.1±112.4 versus 263.7±131.4 mL; p=0.002), and the mean procedure time (from the start of local anaesthesia to procedure end, defined as the time of last guidewire removal) was significantly shorter in the TRA group (74.1±38.4 versus 83.1±43.5 min; p=0.006). Radiation dose did not differ significantly between the TRA and TFA groups (3.0±2.4 versus 3.2±2.4 Gy, respectively, p=0.22). After multivariable adjustment, contrast use was still less with TRA compared to TFA, a finding that was consistent in US and non-US sites (Table 2). Procedural duration was no longer different between groups after multivariable adjustment, although a significant interaction was present such that procedural duration was shorter with TRA at US sites but not at non-US sites. Radiation dose did not differ significantly between groups in the adjusted model.
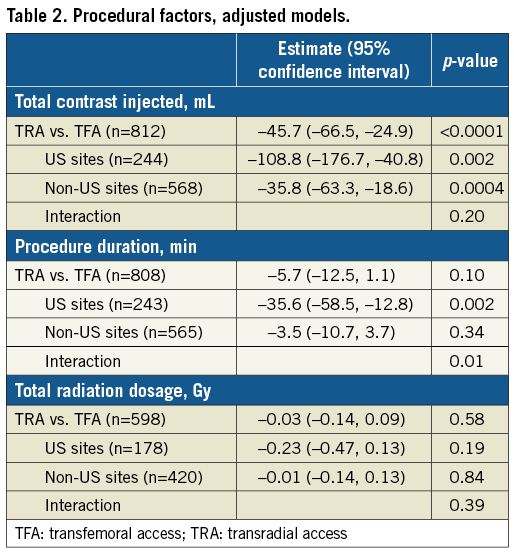
CLINICAL OUTCOMES
At 30 days, the composite outcome measure of death, stroke, or MI had occurred in 6.0% and 4.5% of patients in the TRA and TFA groups, respectively (p=0.35) (Table 3). The rates of the individual components of this endpoint and stent thrombosis also did not differ significantly between groups (Table 3). Any bleeding at 30 days by both the TIMI and BARC scales was numerically lower with TRA compared with TFA; however, differences in clinically important bleeding (TIMI major or minor or BARC 3-5) were not statistically significant (Table 3). The 30-day relative rates of BARC 2-5 bleeding were similar with TRA versus TFA in patients undergoing LM PCI with bivalirudin (1.7% versus 6.2%, respectively, HR [95% CI]=0.27 [0.04, 2.04]) and with heparin only (3.7% versus 5.5%, respectively, HR [95% CI]=0.67 [0.29, 1.57]) (pinteraction=0.42). Procedural vascular complications occurred in 2 (0.8%) TRA and 16 (2.3%) TFA patients (p=0.13).
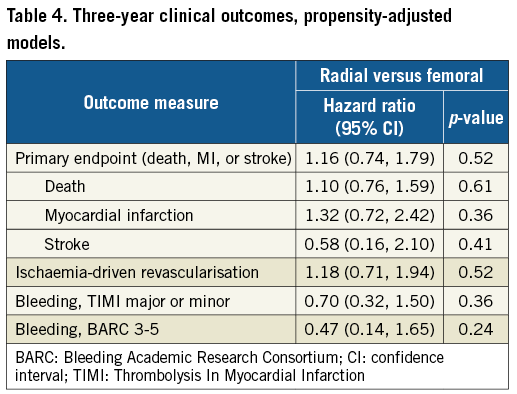
At three years, the primary composite outcome of death, stroke, or MI had occurred in 15.7% and 14.8% of patients in the TRA and TFA groups, respectively (p=0.72) (Table 3, Figure 1). There were no significant differences between the groups in any of the components of the primary endpoint, ischaemia-driven revascularisation, or stent thrombosis (Table 3, Figure 1). Any bleeding at three years by both the TIMI and BARC scales was marginally less with TRA compared to TFA, although the differences in major bleeding did not reach statistical significance (Table 3, Figure 1). After multivariable adjustment, no significant differences were observed between TRA versus TFA for the primary endpoint or any of the major ischaemic or bleeding outcome measures (Table 4).
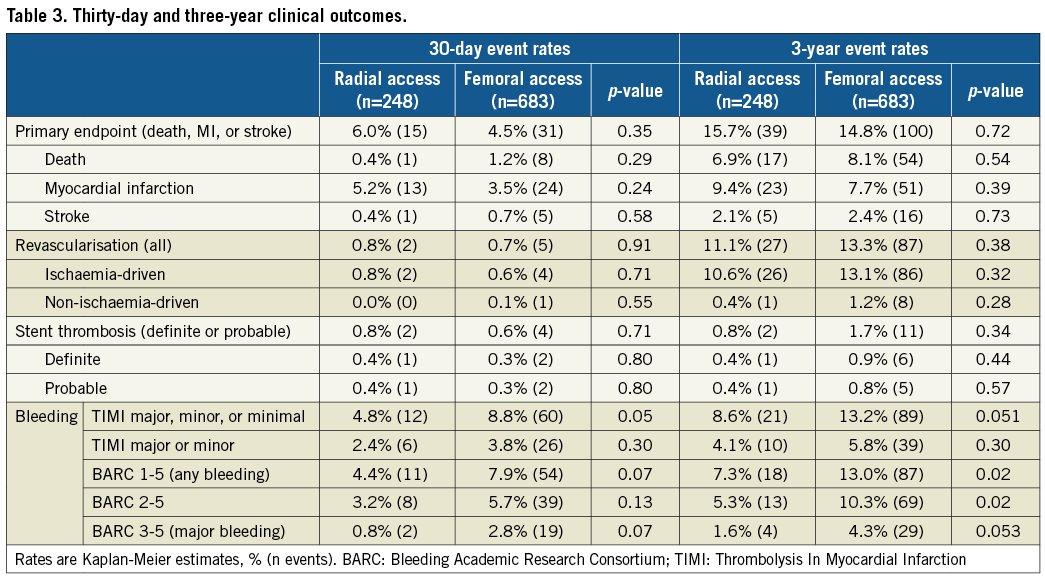
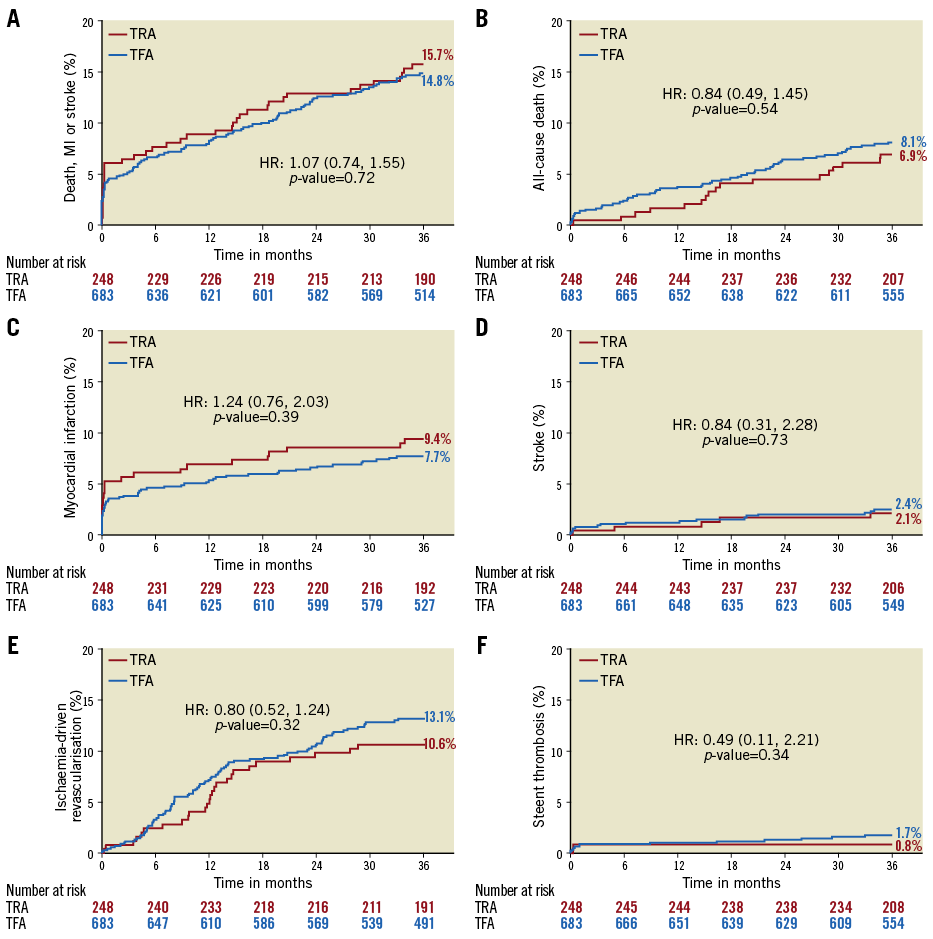
Figure 1. Kaplan-Meier time-to-first-event rates according to transradial or transfemoral access. A) Primary endpoint (death, myocardial infarction or stroke). B) All-cause death. C) Myocardial infarction. D) Stroke. E) Ischaemia-driven revascularisation. F) Definite or probable stent thrombosis.
Discussion
The major findings from the present analysis examining outcomes of PCI for unprotected LM disease according to different routes of vascular access from the contemporary EXCEL trial are that: (i) TRA was used in approximately one quarter of patients, with considerable regional variation and differences in baseline characteristics compared to TFA, and (ii) after multivariable adjustment to account for these differences, there were no significant differences in the three-year risk of ischaemic events or major bleeding in patients undergoing PCI with TRA versus TFA. The present study thus supports the safety and efficacy of TRA for LM PCI.
While the utilisation of TRA has been increasing15, its overall use remains relatively low, particularly in the USA and especially in complex procedures, consistent with our finding that TRA was used in only 26.6% of LM PCI procedures performed in the trial between 2010 and 2014, including only 7.4% of patients enrolled from US sites. Patients for whom TRA was chosen also tended to be lower risk than those in whom TFA was used, being younger, more likely having stable angina and preserved renal function, and having treatment of fewer lesions and vessels. Contrary to previous reports16, total procedure time was not greater with TRA, and in fact compared with TFA was shorter in the USA (but not elsewhere), perhaps reflecting differences in case complexity. Similarly, radiation exposure did not differ significantly between groups, a finding that is also contrary to earlier reports16. These discrepancies between the present and earlier studies may reflect increasing operator experience and greater proficiency with TRA over time, or they may be unique to LM PCI. Finally, the amount of contrast administered was lower with TRA than TFA, even after multivariable adjustment for patient and lesion complexity, which might be related to the fact that the guiding catheter used in TRA was smaller than in TFA. This finding further supports the feasibility of TRA in LM PCI and is consistent with previous reports of reduced acute kidney injury with TRA compared to TFA17. The fact that three-year clinical outcomes did not differ significantly between patients treated with TRA versus TFA implies that TRA utilisation did not impair the quality of the revascularisation or the long-term prognosis.
In most prior studies bleeding complications after PCI have been more frequent with TFA than TRA16, and have been associated with increased morbidity and mortality18-20. Although bleeding was numerically less frequent after TRA in EXCEL, after multivariable adjustment, there was no significant difference in major bleeding between TRA and TFA (TIMI major or minor, or BARC types 3-5). Similarly, while there were fewer vascular complications with TRA compared to TFA, the difference did not reach statistical significance. The lack of significance may be attributed to insufficient statistical power due to low event rates. Alternatively, the greater use of bivalirudin with TFA compared to TRA may have mitigated the bleeding advantage of TRA21-23. The absence of a substantial difference in major bleeding complications between TRA and TFA may also underlie the similar mortality rates observed in the two groups in the present study, in contrast to reduced rates of bleeding and mortality with TRA in some prior trials in patients with acute coronary syndromes24. Regardless of the mechanisms, LM PCI with TRA and TFA was found to result in comparable three-year rates of event-free survival in the EXCEL trial, supporting the feasibility, safety and efficacy of LM PCI with either vascular access route in patients with low and intermediate SYNTAX scores. An appropriately powered dedicated randomised trial would be required to determine whether there are modest differences favouring either TRA or TFA for this high-risk patient cohort.
Limitations
The choice of vascular access was not randomised and was left to the operator’s best judgement. The present post hoc analysis should thus be considered hypothesis-generating. The EXCEL trial was not powered to examine differences in clinical outcomes according to the access site (TRA versus TFA). TFA patients were older and higher risk (greater proportions of chronic kidney disease and acute coronary syndromes, and more often requiring prophylactic haemodynamic support), and had more complex coronary anatomy and disease (greater number of vessels and lesions treated, and more frequent planned two-stent LM strategy). Although these variables were included in our multivariable models, we cannot rule out the presence of unmeasured confounders. Further studies are required to examine outcomes in subgroups of patients with LM disease, such as those with and without distal bifurcation involvement or heavy calcification. We also did not collect detailed data on operator volume or expertise with TRA versus TFA, and further studies are required to determine whether these factors have a major impact on the outcomes of LM PCI.
Conclusions
In conclusion, in patients undergoing LM PCI in the contemporary EXCEL trial, the choice of TRA versus TFA varied considerably across regions, and TRA was only used in approximately one quarter of cases. Nonetheless, TRA was safe and efficacious compared with TFA and may be considered appropriate for LM PCI when performed by suitably experienced operators.
| Impact on daily practice In the EXCEL trial, transradial access (TRA) and transfemoral access (TFA) for percutaneous coronary intervention of left main coronary artery disease (LMCAD) were associated with comparable early and late clinical outcomes. Both TRA and TFA appear to be safe and effective for the treatment of LMCAD. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
The EXCEL trial was sponsored by Abbott Vascular.
Conflict of interest statement
D. Kandzari reports receiving consulting fees from Medtronic and Boston Scientific, and grant support from Abbott Vascular, Medtronic, Boston Scientific, Biotronik, and Medinol. R. Mehran reports: institutional research grant support from Eli Lilly/Daiichi Sankyo, Inc., Bristol-Myers Squibb, AstraZeneca, The Medicines Company, OrbusNeich, Bayer, CSL Behring, Abbott Laboratories, Watermark Research Partners, Novartis Pharmaceuticals, Medtronic, AUM Cardiovascular, Inc., Beth Israel Deaconess Medical Center; executive committee - Janssen Pharmaceuticals, Osprey Medical Inc.; data safety monitoring board - Watermark Research Partners; consulting - Medscape, The Medicines Company, Boston Scientific, Merck & Company, Cardiovascular Systems, Inc. (CSI), Sanofi USA, LLC, Shanghai BraccoSine Pharmaceutical Corp., AstraZeneca; equity - Claret Medical Inc., Elixir Medical Corporation. N. Lembo reports receiving fees for lectures from and serving on advisory boards for Abbott Vascular, Boston Scientific, and Medtronic. A. Banning reports institutional sponsorship for a fellowship from Boston Scientific and lecture fees from Boston Scientific and Abbott Vascular, and being partially funded by the NHS Oxford NIHR Biomedical Research Centre. B. Merkely reports lecture fees and grant support for his institution from Abbott. A.P. Kappetein is an employee of Medtronic. J. Sabik reports receiving fees for serving on advisory boards from Medtronic (cardiac surgery) and the Sorin Group, training fees from Medtronic, and research funding from Abbott and Edwards Lifesciences. P.W. Serruys reports receiving consulting fees from Abbott, Biosensors, Cardialysis, Micell Technologies, Medtronic, Sinomed Science Technologies, Stentys France, Svelte Medical Systems, Philips/Volcano, St. Jude Medical, and Xeltis. G. Stone reports a grant from The Medicines Company to the Cardiovascular Research Foundation, during the conduct of the study; personal fees from Medical Development Technologies, St. Jude, Ablative Solutions, Claret, Sirtex, Matrizyme, Amaranth, BackBeat Medical, Miracor, Neovasc, V-Wave, Shockwave, VALFIX, TherOx, Reva, Vascular Dynamics, and Robocath; other from Aria, Biostar family of funds, MedFocus family of funds, Ancora, Cagent, Qool Therapeutics, SpectraWave, and Caliber, outside the submitted work; his employer, Columbia University, receives royalties for sale of the MitraClip. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
Supplementary Figure 1. Relative use of the transradial versus the transfemoral arterial access according to country/region.
Supplementary Table 1. Angiographic and procedural characteristics.
To read the full content of this article, please download the PDF.

