Abstract
Aims: Factors predicting outcomes after MitraClip implantation are not well defined. We aimed to report the influence of baseline renal function on short-term outcomes of patients enrolled in the investigator-initiated German transcatheter mitral valve interventions (TRAMI) registry.
Methods and results: Twenty participating German centres prospectively included 778 patients (mean age 76.0 years [71-81], 38.8% female gender) at high surgical risk (mean logistic EuroSCORE 20% [12-32%]) undergoing TMVR with the MitraClip for the treatment of symptomatic functional (70%) or degenerative (30%) mitral valve regurgitation (FMR, DMR). The patients were stratified according to renal function before clip implantation. The prevalence of moderate to severe renal impairment (glomerular filtration rate [GFR] <60 ml/min) was 62.7% (37.3%, normal renal function [GFR >60 ml/min]; 49.6%, moderate renal impairment [GFR 30-60 ml/min]; 13.1%, severe renal impairment [GFR <30 ml/min]). TMVR was successfully completed in 98.2% of cases; acute procedural failure, in-hospital and 30-day mortality rates were 1.8%, 2.3% and 4.4%, respectively. Acute procedural failure and mortality rates (in-hospital, 30-day) were significantly higher in patients with severe renal impairment (5.9%, 7.8%, 14.1%), as compared to patients with moderately (1%, 1.3%, 3.0%) or mildly impaired to normal (1.4%, 1.7%, 2.9%) renal function (p<0.0001). Following Cox regression analysis, the prevalence of severe renal impairment at the time of TMVR was the only predictor for increased 30-day mortality rates (hazard ratio 3.42, 95% confidence interval 1.88-6.2; p<0.0001).
Conclusions: Renal function at the time of interventional mitral valve repair with the MitraClip system is a strong predictor for procedural outcomes. Patients with severe renal impairment have a more than threefold increased risk for acute procedural failure, in-hospital death and 30-day mortality.
Introduction
As the general population is undergoing a substantial demographic shift in age, mitral valve regurgitation (MR) has become a typical disease of the elderly patient with complex comorbidities1. The data of the European Heart Survey suggest that more than 30% of patients with relevant valvular heart diseases do not undergo surgery, predominantly because of severely impaired left ventricular function, advanced age, or comorbidities1,2. In recent years, different techniques for transcatheter mitral valve repair (TMVR) have been developed3, and, according to the results of the prospective randomised EVEREST trials (Endovascular Valve Edge-to-Edge REpair STudy), interventional edge-to-edge repair of MR with the MitraClip (MC) system has been established as an alternative treatment option to open heart surgery in selected patients with MR4. Meanwhile, the safety and feasibility of TMVR with the MitraClip has been shown in different patient populations, with reported success rates of up to 99%4. This technique has been widely adapted for clinical use, with more than 12,000 documented implants worldwide at this time.
Despite increasing evidence supporting this therapeutic approach, factors affecting procedural outcomes with the MitraClip are still unclear. In particular, the impact of renal function on procedural outcome has not been systematically studied in a large cohort of patients. The German transcatheter mitral valve interventions (TRAMI) registry is the largest series of consecutive, unselected patients undergoing MitraClip treatment. Here we report the influence of baseline renal function on short-term prognosis 30 days after MitraClip implantation.
Methods
THE GERMAN TRAMI REGISTRY
The aims and scope of the German TRAMI registry have been published previously5,6. In brief, the investigator-initiated registry was launched in August 2010 at participating German centres offering percutaneous mitral valve therapies. The purpose of the registry is to assess prospectively the safety and efficacy of mitral valve interventions for the treatment of either mitral valve stenosis or MR. Data are collected by using an internet-based standardised electronic case report form. Prospective data enrolment was started in August 2010. Follow-up (FU) was scheduled at 30 days, one, three and five years after the procedure (prospective section).
Additionally, centres were encouraged to enter retrospectively all patients who had been treated with the MitraClip system since January 2009 (retrospective section).
PATIENTS
Here we report data from the prospective section of the TRAMI registry, including patients with symptomatic, severe MR planned for the MitraClip procedure, treated at 20 German centres. Follow-up data were evaluated for at least 30 days after MitraClip procedures. MACCE was defined as major adverse cardiac or cerebrovascular events including stroke, TIA and myocardial infarction. Patients were subdivided according to renal function at the time of planned procedures. Renal function was defined using the documented glomerular filtration rate (GFR) following the Modification of Diet in Renal Disease (MDRD) formula: mildly impaired or normal renal function is defined as GFR >60 ml/min, moderately impaired renal function as GFR 30-60 ml/min, and severely impaired renal function as GFR <30 ml/min.
All patients had to provide written informed consent before study inclusion. The study was approved by local ethics committees and is in accordance with the Declaration of Helsinki.
INTERVENTIONAL EDGE-TO-EDGE REPAIR OF MR
All procedures were performed under general anaesthesia using fluoroscopy and transoesophageal echocardiographic guidance. In all cases, no contrast agent was used for MC placement.
To perform MC placement, the right atrium is accessed via the left or right femoral vein. After transseptal puncture a wire is passed into the left atrium to allow guide catheter introduction. After positioning the guide in the mid left atrium, the clip delivery system is brought forward into the guide catheter and advanced into the left atrium. Using echocardiographic and fluoroscopic guidance, the clip is moved until the device is centred over the visible mitral regurgitant orifice. Thereafter, the clip arms are opened and oriented perpendicular to the long axis of the leaflet edges and the clip is advanced into the left ventricle below the mitral leaflet edges. For the grasping process, the clip is closed to approximately 120° and pulled back until the mitral leaflets are captured in the arms of the clip; after ensuring proper leaflet insertion, the grippers are lowered and the clip is closed. At this point, the degree of MR reduction is checked with echocardiography. If functional results are appropriate and relevant MV stenosis is excluded with echo, the clip is released from the delivery system and the delivery system and guide catheter are withdrawn4. The severity of MR was graded into three stages as I (mild), II (moderate) and III (severe). Procedural success was defined as post-procedural MR of less than or equal to grade II. The number of clips which were implanted to reach procedural success was left to the discretion of the treating physician. Before clip release, pulsed wave and continuous wave Doppler measurements of MV inflow velocities were performed to exclude clinically relevant MV stenosis (defined as mean pressure gradient >5 mmHg)4.
STATISTICAL ANALYSIS
All statistical analyses were performed independently at the Institut für Herzinfarktforschung, Ludwigshafen, Germany. Categorical variables are presented as absolute numbers and percentages and are compared by Cochran-Armitage test for trend. Continuous data were expressed as mean values±standard deviation or median (with interquartile ranges) and are compared by Jonckheere-Terpstra test. All tests were two-tailed and p-values <0.05 were considered significant. The cumulative survival in relation to GFR was estimated by the Kaplan-Meier method. Survival in groups was compared using the log-rank test. Multivariable Cox regression was performed to analyse the influence of relevant variables on 30-day mortality, including female gender, age >75 years, aetiology of MR, left ventricular ejection fraction (LVEF) <30%, severe tricuspid regurgitation, systolic pulmonary arterial pressure (sPAP) and atrial fibrillation in addition to GFR <30 ml/min for adjustment. The SAS statistical package version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for the computations.
Results
DEMOGRAPHIC BASELINE PARAMETERS
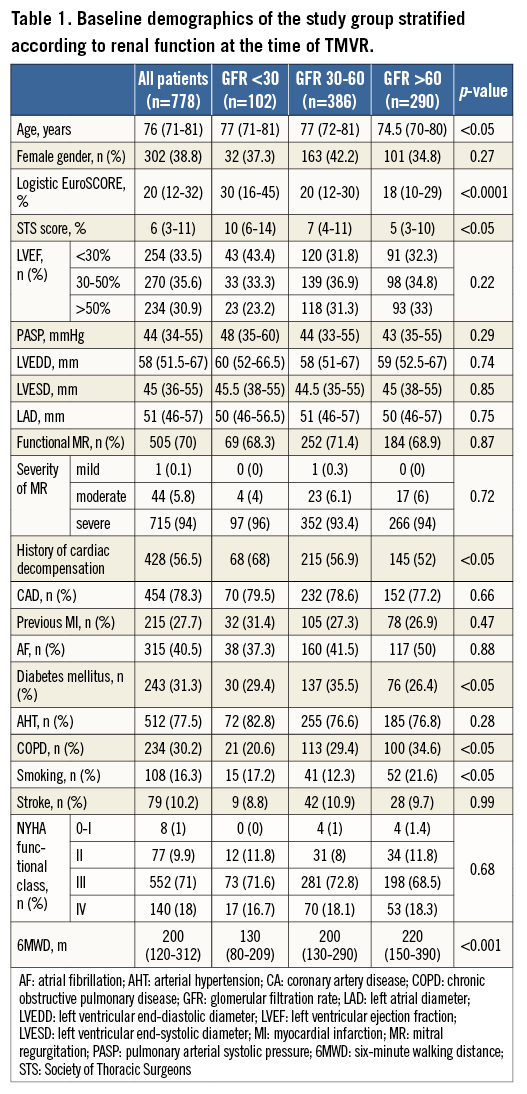
From August 2010 to October 2013, 778 consecutive patients (mean age 76.0 years [71-81], 38.8% female gender) with documented GFR judged to be not amenable for open heart surgery (mean logistic EuroSCORE 20% [12-32%]) by Heart Team decisions (61.9%), cardiothoracic surgeons (1.4%), or cardiologists alone (36.6%), undergoing TMVR with the MitraClip system due to functional (FMR, 70%) or degenerative (DMR, 30%) valve disease, were prospectively included. Reflecting current clinical practice of patient selection for MitraClip therapy7, the majority of patients presented in the advanced stages of ischaemic (78.3%) or non-ischaemic (11%) heart failure (HF), as reflected by predominantly high functional NYHA classes (III to IV in 89% of cases), increased levels of NT-proBNP (3,500 pg/ml [range 1,579-6,940 pg/ml]), elevated systolic pulmonary artery pressure (sPAP) of 44 mmHg (range 34-55 mmHg), and reduced six-minute walking distances before clip implantation (200 m [range 120-312 m]). FMR was predominantly caused by ischaemic heart disease with reduced left ventricular ejection fraction (LVEF) <50% in 69.1% of the overall population, including 33.5% of patients with LVEF <30% (Table 1).
Information on in-hospital outcomes was obtained in all patients (n=778, 100%); 30-day outcome data were completed in 96% of cases (n=728).
PREVALENCE OF RENAL IMPAIRMENT AND COMPARISON BETWEEN GROUPS
Mildly impaired or normal renal function (GFR >60 ml/min) was documented in 290 patients (37.3%), and moderate to severe renal impairment (GFR 30-60 ml/min) was prevalent in 488 patients (62.7%), including 386 patients (49.6%) with moderately impaired and 102 subjects (13.1%) with severely impaired renal function (GFR <30 ml/min).
When comparing groups according to renal function (severe, moderate, mild - normal; Table 1), patients with severe renal impairment more often had a history of cardiac decompensation within the last six months before interventions (68%, 56.9%, 52%; p<0.05) and were significantly more often judged by treating physicians to be at higher surgical risk than the other patient groups (71.6%, 58.0%, 57.6%; p<0.05), as reflected by an increased logistic EuroSCORE (30% [16-45%], 20% [12-30%], 18% [10-29%]; p<0.0001) and Society of Thoracic Surgeons (STS) score (10% [6-14%], 7% [4-11%], 5% [3-10%], p<0.05).
According to documented risk factors, the calculated risk scores were predominantly increased due to renal dysfunction at the time of TMVR, and patients with normal renal function were significantly more often smokers (active or former) (17.2%, 12.3%, 21.6%, p<0.05) and more often suffered from chronic obstructive lung disease (20.6%, 29.4%, 34.6%, p<0.05). Concerning functional parameters, patients with GFR <30 ml/min presented with significantly lower six-minute walking distances (6MWD) before TMVR as compared to other groups (mean 130 m [80-209 m], 200 m [130-290], 220 m [150-390]; p<0.001), whereas NYHA functional classes before TMVR were not statistically different among the groups (p>0.05).
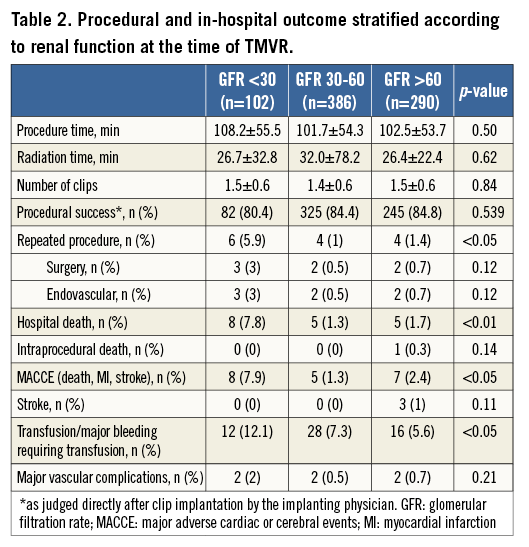
IN-HOSPITAL OUTCOMES AND PROCEDURAL SUCCESS RATES
Acute procedural success (post-procedural MR ≤II) was achieved in 98.2% of cases, 12 interventions (1.5%) were aborted before MitraClip implantation, and clip embolisation did not occur in any of the treated patients. In 14 cases (1.8%), the procedure was judged as procedural failure by the treating physicians, and 21 patients (2.7%) had a remaining severe MR after MitraClip implantation. During index hospitalisation, seven (0.9%) patients had to undergo urgent mitral valve surgery, and four (0.5%) patients underwent repeat MitraClip implantation. After hospital discharge, six patients (1.2%) had to undergo a second MitraClip procedure within 30 days because of relapse of mitral regurgitation. The in-hospital mortality rate was 2.3% (n=18), including one intraprocedural death, and the 30-day mortality rate was reported as 4.4% (n=32).
IMPACT OF RENAL IMPAIRMENT ON PROCEDURAL SUCCESS AND 30-DAY OUTCOMES
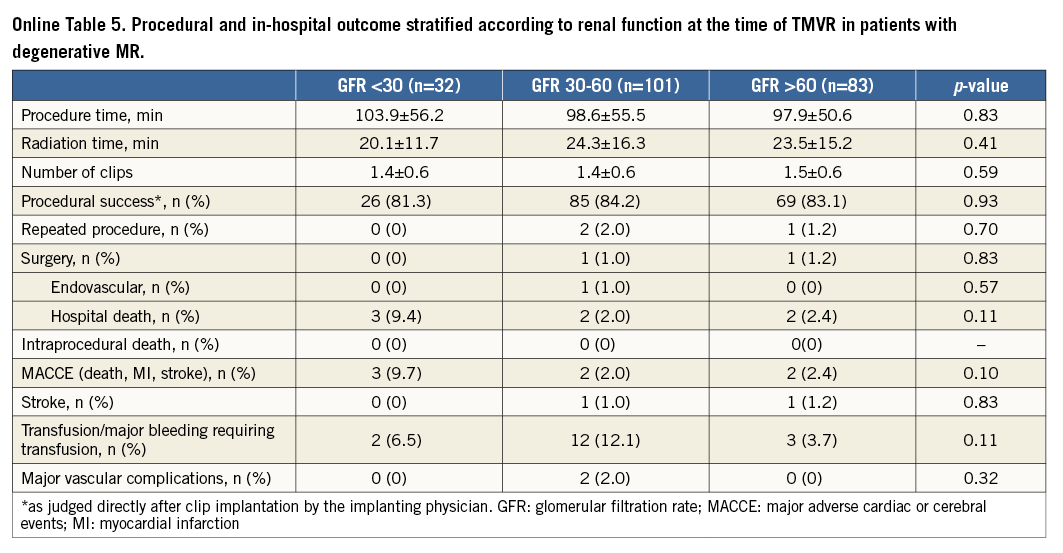
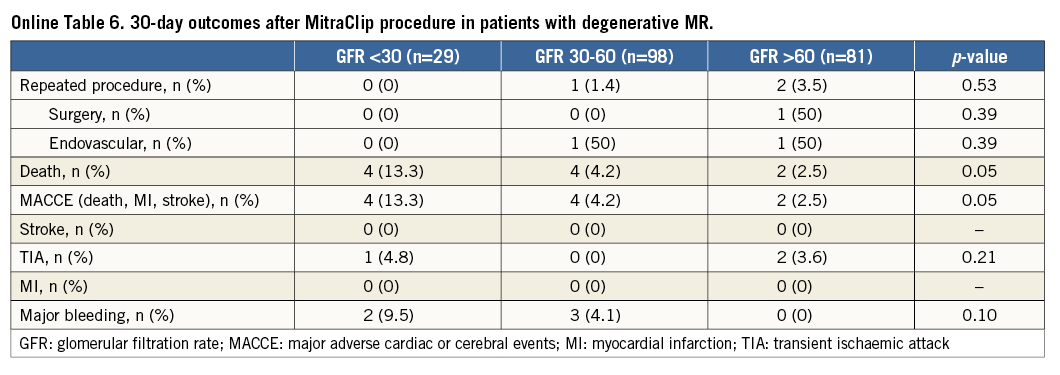
When comparing the groups according to the documented renal function at the time of MitraClip implantation (severe, moderate, mild - normal), patients with severe renal impairment had significantly worse outcomes concerning acute procedural failure rates (5.9%, 1%, 1.4%; p<0.001), in-hospital mortality (7.8%, 1.3%, 1.7%; p<0.0001) and 30-day mortality rates (14.1%, 3.0%, 2.9%; p<0.0001) (Figure 1). This resulted in significantly higher in-hospital and 30-day MACCE rates (death, myocardial infarction and stroke) in patients with severely impaired renal function (7.9%, 15.4%) as compared to the groups with moderate renal impairment (1.3%, 3.0%) and mildly impaired or normal renal function (2.4%, 3.7%) (p<0.0001) (Figure 2, Table 2).
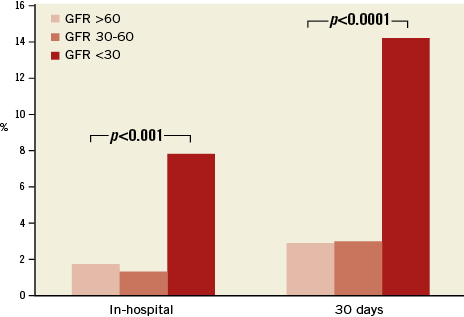
Figure 1. In-hospital and 30-day mortality rates of patient groups stratified according to renal function. GFR: glomerular filtration rate
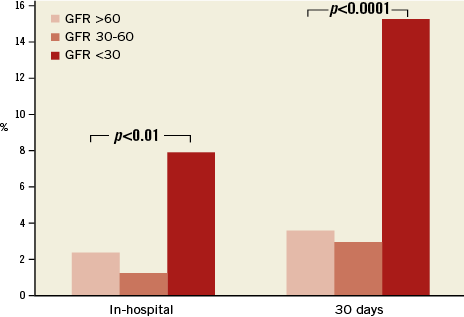
Figure 2. In-hospital and 30-day MACCE rates of patients stratified according to renal function. GFR: glomerular filtration rate
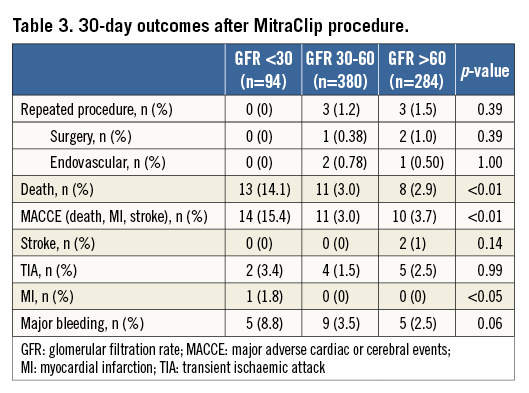
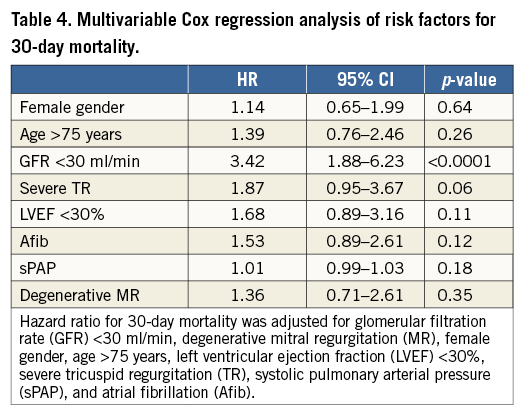
Kaplan-Meier analysis confirmed significantly worse survival of patients with GFR <30 ml/min as compared to the other groups (Figure 3) (pLog-rank<0.0001). After Cox regression analysis, only severe renal impairment (GFR <30 ml/min) at the time of MitraClip implantation was significantly associated with an increased mortality risk during 30 days after MitraClip procedures with a hazard ratio (HR) of 3.42 (95% confidence interval [CI] 1.88-6.2; p<0.0001) (Table 3, Table 4).
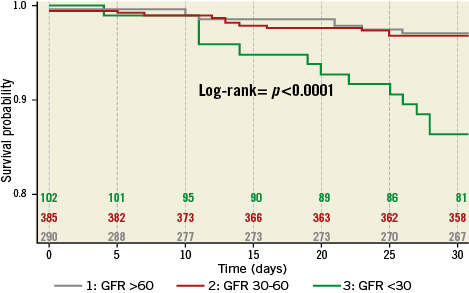
Figure 3. Kaplan-Meier curves for 30-day survival. GFR: glomerular filtration rate
The majority of patients (67.2%) had a substantial clinical benefit with regard to improvement of NYHA class to 0-II, which was independent from renal function (Figure 4).
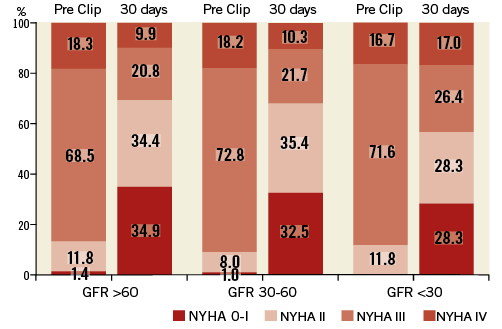
Figure 4. NYHA functional classes before and 30 days after MitraClip treatment. GFR: glomerular filtration rate; NYHA: New York Heart Association
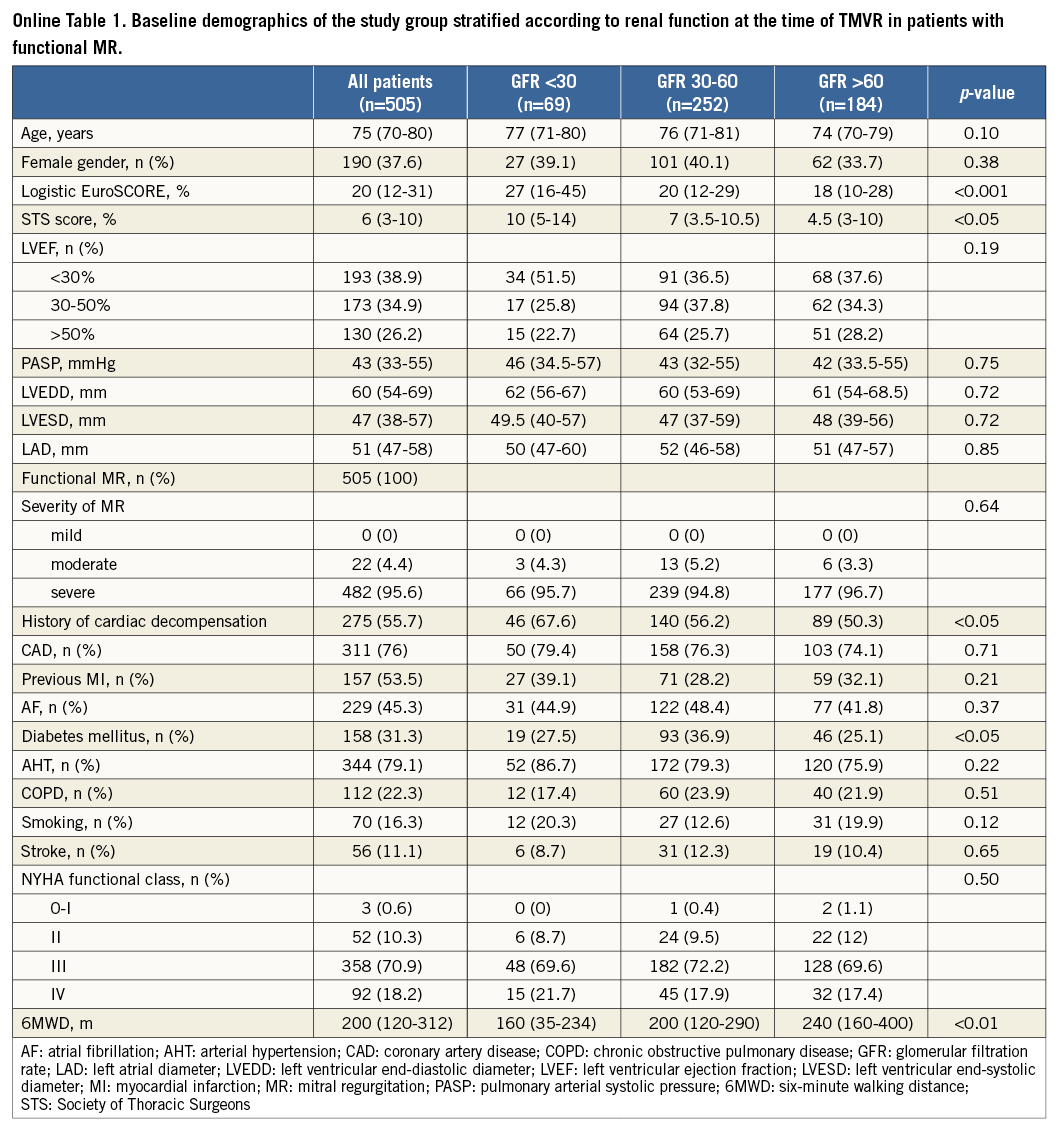
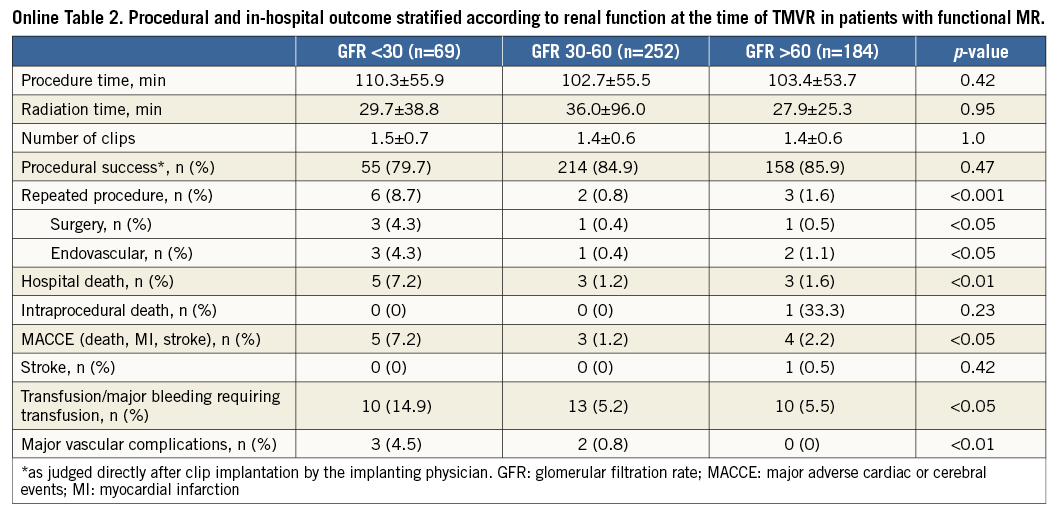
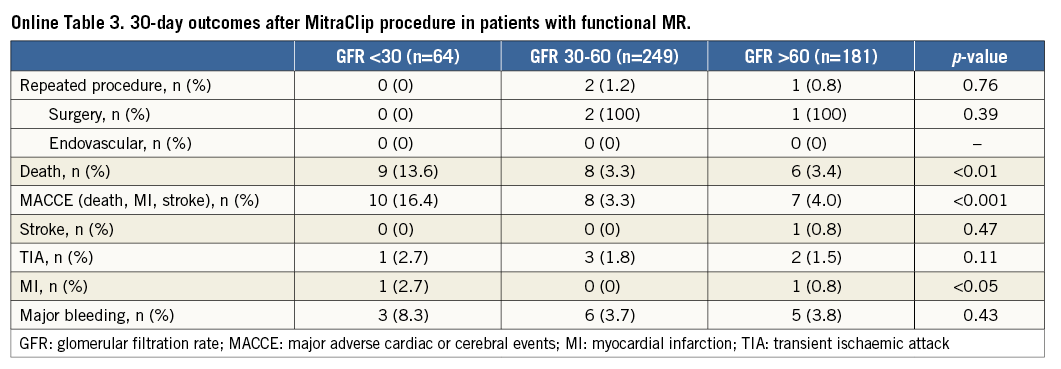
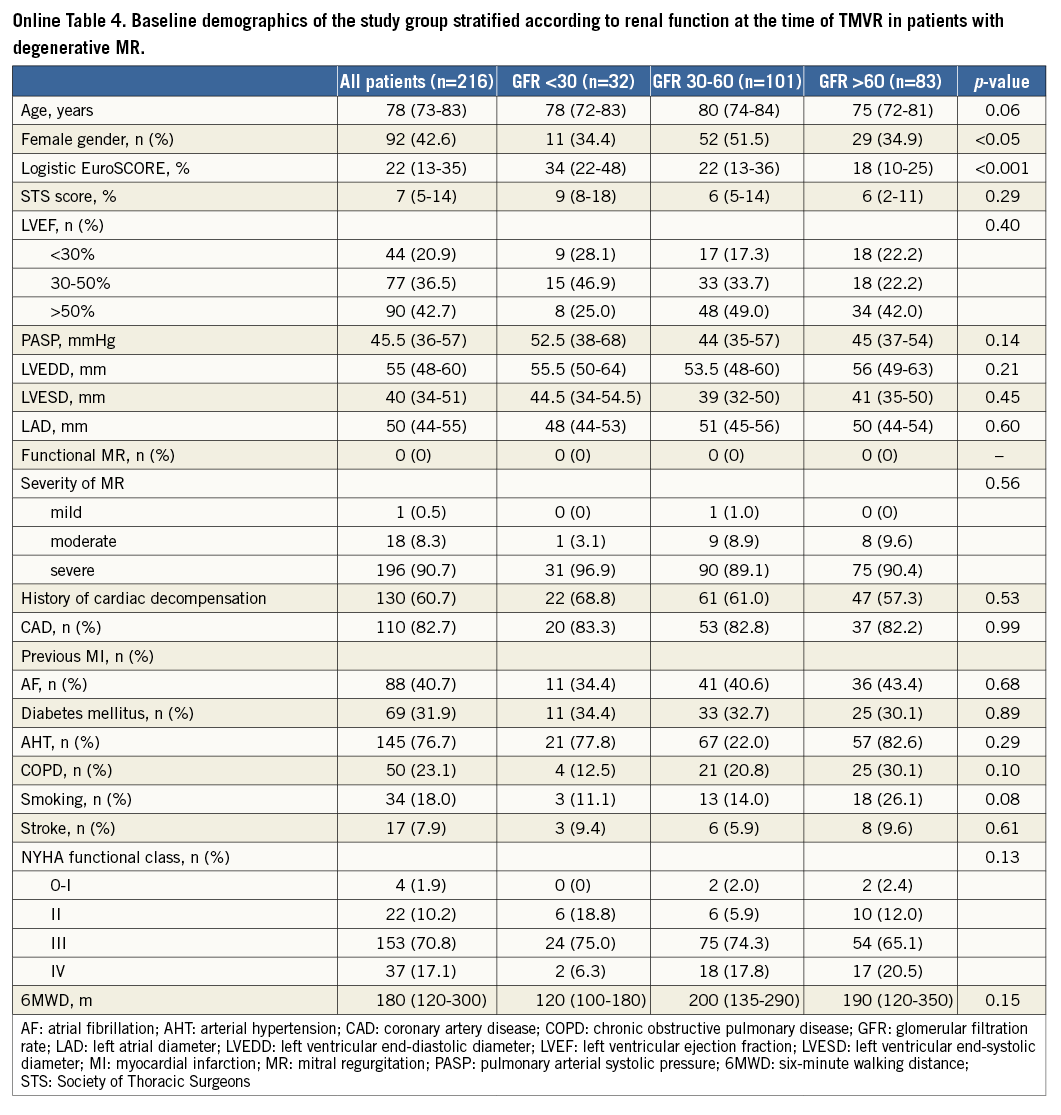
When analysing the impact of renal impairment on 30-day outcome separately in patients with functional and degenerative MR, we found statistically significant differences in mortality and MACCE rates at 30 days in patients with functional and degenerative MR depending on renal function at baseline (Online Table 1-Online Table 6).
Discussion
To the best of our knowledge, this is the first large study to analyse the impact of renal function at the time of TMVR with the MitraClip system on procedural and 30-day outcomes. In this prospective registry of unselected patients undergoing mitral valve procedures, we found a strong impact of renal function at the time of MitraClip treatment on acute and short-term patient outcomes. Patients with severe renal impairment had a more than threefold increased risk of acute procedural failure, in-hospital and 30-day death and MACCE.
ASSOCIATION OF RENAL FUNCTION WITH CARDIOVASCULAR MORBIDITY AND MORTALITY
Renal dysfunction has been proven to be an independent marker of cardiovascular risk and morbidity in different clinical settings and in different patient cohorts8. In an unselected population without known coronary artery disease, the prevalence of reduced GFR and of albuminuria was independently associated with an increased risk of ischaemic cardiovascular events, even after correction for traditional risk factors9. Holzmann et al identified renal impairment as predictive of recurrent myocardial ischaemia and the development of symptomatic HF in a cohort of patients undergoing isolated coronary artery bypass grafting10,11. In a cohort of 77 patients undergoing transcatheter aortic valve replacement (TAVR), Sinning et al recently reported that patients with impaired renal function at baseline are at increased risk of death 30 days after TAVR (HR 3.9, 95% CI: 1.6-9.5; p=0.002)12. A similar negative effect on survival was demonstrated by Thourani et al for patients undergoing surgical AVR13.
Nakazato and co-workers found comparable results in patients undergoing surgical mitral valve repair or replacement during five years of FU. The authors reported significantly increased MACE rates of 57.2% in patients with GFR <30 ml/min as compared to 29.6% in patients with normal renal function14.
In concordance with Sinning et al, we found a high prevalence of renal impairment in a high-risk cohort of multi-morbid patients scheduled for interventional treatment of heart valve disease12. The impact of renal function on TMVR-related outcomes, however, has not yet been studied. According to current knowledge, the procedural risk of MitraClip therapy is very low, ranging between 0-3.8% for in-hospital and 3.4-7.7% for 30-day mortality7,15,16, which is in line with our findings (in-hospital deaths 2.3%, death at 30 days 4.4%). These studies, however, were not focused on the identification of factors affecting clinical outcomes after clip implantation. The results of the present study, derived from a large unselected cohort of patients with different stages of renal function undergoing MitraClip treatment, clearly emphasise the impact of renal function on patient outcomes. In line with studies on other complex cardiac interventions, we found a significantly increased risk of procedural failure and short-term mortality in subjects with severe renal impairment, which was increased more than threefold as compared to patients with GFR >30 ml/min (HR 3.4, 95% CI: 1.88-6.2; p<0.0001).
RENAL IMPAIRMENT: MARKER OR MEDIATOR OF CARDIOVASCULAR OUTCOMES?
In patients with different manifestations of cardiovascular disease, renal dysfunction seems to be both a marker and a mediator of adverse outcomes17,18. It plays a crucial role in the pathophysiology of HF and represents the most important comorbidity in this fragile patient population19. In conservatively treated HF patients, the prevalence of renal impairment is known to be associated with worse survival, a higher risk for re-hospitalisation, and worsening of HF-related symptoms20,21. Importantly, impaired renal function negatively impacts on patient outcomes independently from other established risk factors such as LV function or severity of HF symptoms22. In concordance with the results of the recently published ACCESS EU registry23, the patients in the present study underwent TMVR for the treatment of advanced stages of symptomatic HF (89% NYHA Class ≥III), with a high prevalence of FMR (70%). In this selected population, renal impairment increases mortality rates with worse survival in patients with severely impaired renal function, which is in line with other studies on HF patients24. Of note, this effect seems additionally enhanced if these patients undergo complex cardiac interventions such as TMVR, as shown with the reported outcome data. Future studies are warranted to investigate the influence of renal function-restoring interventions before TMVR on acute and intermediate procedural outcomes.
Limitations
There are several limitations to this study. First, all data were exclusively site-reported and the authors cannot vouch for the integrity of all the data presented. However, this limitation applies to every multicentre registry. Second, follow-up time in the present study was limited; we cannot exclude that patients with GFR >30 ml/min will “catch up” with mortality rates over time. A multivariate analysis of several factors possibly affecting clinical outcome was not carried out because of the low endpoint count. The strength of our database is the monitoring of the current use of the MitraClip in a real-world setting in quite a large cohort of prospective patients.
Conclusion
Renal function at the time of interventional mitral valve repair with the MitraClip system is a strong predictor for procedural outcomes. Patients with severe renal impairment have a more than threefold increased risk of acute procedural failure, in-hospital death and 30-day mortality. Future studies are warranted to investigate the influence of renal function-restoring interventions before TMVR on procedural outcomes.
| Impact on daily practice Factors predicting outcomes after MitraClip implantation are not well defined. The negative influence of reduced renal function on outcomes in different patient cohorts is well known and the impact of renal impairment on outcomes after MitraClip implantation is unknown. Renal function at the time of interventional mitral valve repair with the MitraClip system is a strong predictor for procedural outcomes. Patients with severe renal impairment have a more than threefold increased risk for acute procedural failure, in-hospital death and 30-day mortality. Therefore, the decision process for MitraClip implantation should include consideration of renal function along with other risk factors. |
Funding
The majority of funding for TRAMI is provided by proprietary means of the Stiftung Institut für Herzinfarktforschung (IHF)/Ludwigshafen. Additional funding is provided by Deutsche Herzstiftung e.V. Additional funding for biometrics is provided by Abbott Vascular Deutschland.
Conflict of interest statement
H. Sievert has received study honoraria, travel expenses, consulting fees from Access Closure, AGA, Angiomed, Ardian, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm Medical, Recor, Rox Medical, Sadra, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Viacor, Velocimed, Veryan, and holds stock options from CardioKinetix, Access Closure, Velocimed, CoAptus, Lumen Biomedical and Coherex. W. Schillinger has received honoraria and travel expenses from Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data